
Index ntawm nplooj ntawv ntsiab lus
En Ok Pauv Reform, nyob rau hauv nqe lus no nyob rau hauv lub pH theem pas ua luam dej peb yuav kho cov qhov sib txawv ntawm ph thiab poh nyob rau hauv lub pas dej muaj nuj nqis.
Dab tsi yog pH hauv lub pas dej thiab yuav ua li cas nws qib?

Qhov pH zoo tagnrho txhais li cas rau cov pas dej da dej (7,2-7,4)
Lub ntsiab lus pH sawv cev rau qhov muaj peev xwm hydrogen thiab yog ib qho kev ntsuas uas qhia txog acidity lossis qhov pib ntawm dej.
Yog li ntawd, pH yog hais txog lub peev xwm ntawm hydrogen, tus nqi uas sib haum mus rau qhov concentration ntawm hydrogen ions hauv dej hauv koj lub pas dej ua ke thiab yog li ntawd cov coefficient uas qhia txog qib acidity lossis theem pib ntawm cov dej. Yog li, pH yog tus saib xyuas qhia qhov concentration ntawm H + ions hauv dej, txiav txim siab nws cov kua qaub lossis cov cim tseem ceeb.
Scale ntawm pH qhov tseem ceeb ntawm lub pas dej da dej
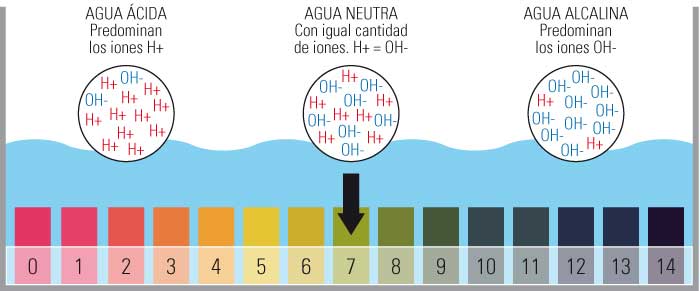

Cov txiaj ntsig dab tsi hauv pas dej da dej pH ntsuas ntsuas suav nrog?
- Qhov ntsuas pH suav nrog cov nqi ntawm 0 txog 14.
- Tshwj xeeb yog 0 qhov acidic tshaj plaws, 14 qhov yooj yim tshaj plaws thiab muab qhov nruab nrab pH ntawm 7.
- Qhov ntsuas no yog txiav txim los ntawm tus naj npawb ntawm cov dawb hydrogen ions (H +) hauv cov khoom.

Vim li cas peb thiaj xav tau pH?
pH yog ib qho kev ntsuas siv los qhia cov acidity los yog hauv paus ntawm cov kua dej aqueous. Txawm hais tias cov tshuaj aqueous reacts li cov kua qaub los yog lub hauv paus nyob ntawm nws cov ntsiab lus ntawm hydrogen ions (H +).
Txawm li cas los xij, txawm tias cov dej ntshiab huv thiab nruab nrab muaj qee cov hydrogen ions vim nws tus kheej dissociation ntawm dej.
Nws paub tias ntawm qhov sib npaug raws li tus qauv (750 mmHg thiab 25 ° C), 1 L ntawm cov dej ntshiab muaj mole
y
mole
ions, yog li ntawd, dej ntawm tus qauv kub thiab siab (STP) muaj pH ntawm 7.
Yuav ua li cas thaum pH ntawm peb lub pas dej tsis tswj hwm

Paub qhov pH siab ntawm pas dej ua ke thiab ua rau muaj pH siab hauv koj lub pas dej
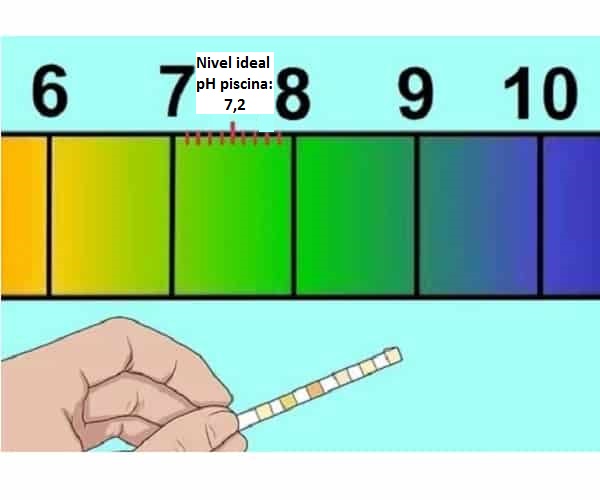
Yuav ua li cas nce pH ntawm lub pas dej thiab yuav ua li cas yog tias nws qis

Yuav ua li cas kom txo tau qhov siab lossis Alkaline pas dej pH
Cov lus qhia txog yuav ua li cas kho pas dej ua ke ntxiv rau pH: dej tu thiab tshuaj tua kab mob

Cov lus qhia tseem ceeb kom paub yuav ua li cas ntxuav lub pas dej

Phau ntawv saib xyuas lub pas dej ua ke nrog dej hauv qhov xwm txheej zoo meej
Acid, nruab nrab thiab alkaline pH qhov tseem ceeb
Kev faib tawm ntawm qhov ntsuas pH tus nqi
Dab tsi yog cov nqi pH

Lub pH nplai mus los ntawm 1 txog 14, nrog pH 7 yog ib qho kev daws teeb meem nruab nrab.
Yog li, nws hloov tawm tias pH yog tus nqi uas tau hais tawm ntawm qhov ntsuas logarithmic nruab nrab ntawm qhov tseem ceeb 0 (tsis tshua muaj acidic) thiab 14 (alkaline heev); Hauv nruab nrab peb pom tus nqi 7 cataloged li nruab nrab.
pH scale universal pH qhia
Nws txhais li cas tias cov khoom muaj acidic lossis alkaline pH?
Dab tsi yog acids thiab bases?
Cov kua qaub thiab cov hauv paus yog cov khoom uas muaj nyob hauv qhov xwm txheej thiab txawv ntawm lawv qib pH, uas yog, los ntawm lawv qib acidity lossis alkalinity. Kev txiav txim siab ntawm seb cov khoom puas yog acidic lossis alkaline yog tswj hwm los ntawm qib acidity lossis alkalinity ntsuas los ntawm pH nplai thiab thaj tsam ntawm 0 ( acidic heev mus rau 14 (tsis tshua muaj alkaline). txawm li cas los xij muaj ntau yam kev lag luam thiab tib neeg siv.
Cov tshuaj acidic yog dab tsi?
- acid pH: pH tsawg dua 7
Nws txhais li cas tias tus nqi pH yog acidic?
- Hais tias ib yam khoom yog acidic txhais tau hais tias nws yog nplua nuj nyob rau hauv H+ (hydrogen ions): pH ntau dua 7
- Vim li no, Acids yog cov khoom uas muaj pH tsawg dua 7. (pH ntawm dej sib npaug li 7, suav tias yog nruab nrab), uas nws chemistry feem ntau muaj cov hydrogen ions ntau thaum ntxiv dej. Lawv feem ntau hnov mob nrog lwm yam tshuaj los ntawm kev poob protons (H+).
Dab tsi yog cov khoom nruab nrab?
- Nruab nrab pH tus nqi: pH sib npaug rau 7-
Nws txhais li cas tias pH tus nqi nruab nrab?
- pH yog ib qho kev ntsuas ntawm acidic/basic dej.
- Qhov ntau yog los ntawm 0 txog 14, nrog 7 yog nruab nrab.
Cov tshuaj alkaline yog dab tsi?
- Cov khoom uas muaj lub hauv paus lossis alkaline pH: pH ntau dua 7.
Nws txhais li cas thaum tus nqi pH yog alkaline?
- Tias ib yam khoom yog alkaline txhais tau hais tias nws tsis zoo hauv H+ (los yog nplua nuj hauv OH hauv paus-, uas neutralize H+).
- Rau tag nrho cov no, Bases, ntawm qhov tod tes, yog cov khoom uas muaj pH ntau dua 7., uas nyob rau hauv aqueous daws feem ntau muab hydroxyl ions (OH-) nyob nruab nrab. Lawv zoo li muaj zog oxidants, uas yog, lawv hnov mob nrog protons los ntawm ib puag ncig nruab nrab.
Qhov sib txawv ntawm pH thiab pOH tus nqi

Lawv cuam tshuam li cas thiab qhov txawv ntawm qhov ntsuas ph thiab poh yog dab tsi?
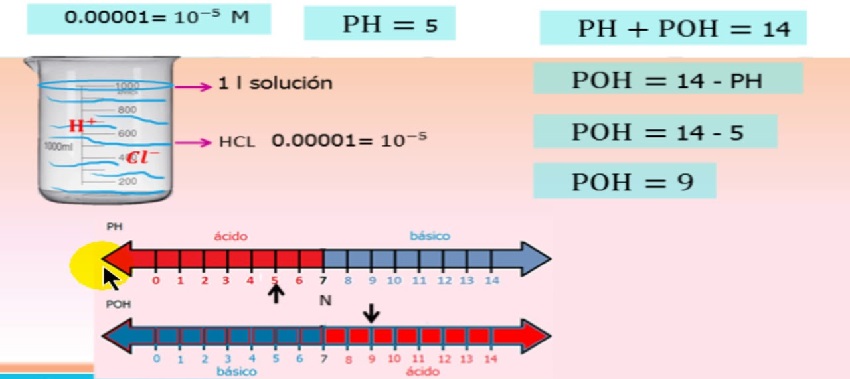
Tau kawg, qhov kev ua ntawm ions nyob ntawm qhov concentration ntawm ion thiab qhov no tau piav qhia hauv kab zauv
Kev sib npaug ntawm pH / poH ion kev ua haujlwm
qhov twg, - kev ua haujlwm ntawm hydrogen ion
- Kev ua haujlwm coefficient ntawm hydrogen ion
- hydrogen ion concentration
Cov dej num coefficient yog kev ua haujlwm ntawm ion concentration thiab mus txog 1 raws li kev daws teeb meem ntau dua thiab ntau dua.
Rau dilute (zoo tagnrho) cov kev daws teeb meem, tus qauv lub xeev ntawm cov kuab tshuaj yog 1,00 M, yog li nws cov molarity sib npaug nws cov dej num.
Yog li, rau feem ntau cov teeb meem uas xav tias cov kev daws teeb meem zoo tshaj plaws, peb tuaj yeem siv lub logarithm mus rau lub hauv paus 10 ntawm molar concentration, tsis yog kev ua haujlwm.
Qhov sib txawv ntawm tus nqi ntawm pH thiab pOH yog dab tsi
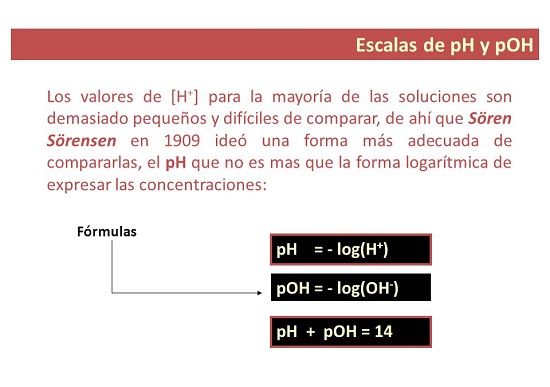
Tus nqi pH li cas yog dab tsi?
- Hauv ib txoj kev, pH yog qhov ntsuas qhov ntawd siv los tsim cov qib ntawm acidity lossis alkalinity ntawm kev daws. Lub "p" sawv cev rau "muaj peev xwm", uas yog vim li cas pH hu ua: muaj peev xwm ntawm hydrogen.
Tus nqi pOH yog dab tsi?
- Rau koj ib feem. pOH yog ib qho kev ntsuas ntawm qhov concentration ntawm hydroxyl ions hauv cov tshuaj. Nws yog qhia raws li lub hauv paus 10 tsis zoo logarithm ntawm hydroxyl ion concentration thiab, tsis zoo li pH, yog siv los ntsuas cov alkalinity theem ntawm kev daws.

Tus nqi pH lossis pOH suav li cas?
Cov mis rau ph scale qhov tseem ceeb yog dab tsi?
- Raws li nws twb paub lawm, nyob rau hauv lub scientific teb, lub pH yog qhov ntsuas de cov ions sab hauv de kev daws teeb meem. Tej zaum koj yuav tau xam pH raws li concentration. Xam cov pH Siv qhov sib npaug ntawm pH: pH = -log[H3O+].
Tus qauv los xam pOH yog dab tsi?
- Ntxiv thiab, tus pOH (los yog OH peev xwm) yog ib qho kev ntsuas ntawm lub hauv paus los yog alkalinity ntawm kev daws. Kuj se siv pH = – log [H3O+] ntsuas qhov concentration ntawm hydronium ions [H3O+].

Cov zauv tseem ceeb los xam pH lossis pOH tus nqi
- pH=-log[H3O+]
- pOH=-log[OH-]
- [H3O+= 10-pH
- [oh-= 10-pOH
- pH + pOH = pKw 14.00 Nws 25°C.
Dab tsi yog qhov txawv ntawm qhov ntsuas pH thiab qhov pOH?
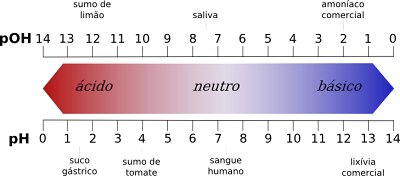
Kev tsis sib xws ntawm cov nqi ntawm pH nplai
- Ntawm ib sab, pH nplai muab cov kua qaub ntawm 1 txog 6 thaum pOH nplai muab cov kua qaub ntawm 8 txog 14.
- Hloov pauv, pH nplai muab qhov tseem ceeb ntawm 8 txog 14, thaum pOH nplai muab qhov tseem ceeb ntawm 1 txog 6.
Logarithm scale kev sib raug zoo ntawm ph thiab pOH nrog lawv cov nqi
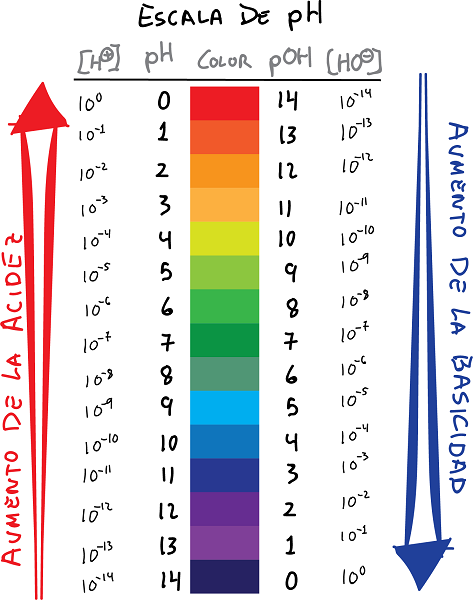
ph thiab pOH nplai kev sib txuas nrog cov xim thiab qhov tseem ceeb
- pH yog lub logarithm ntawm cov concentration ntawm H ions+, nrog lub cim hloov:
- Ib yam li ntawd, txhais pOH Raws li lub logarithm ntawm OH ion concentration-, nrog rau kev hloov pauv: Cov kev sib raug zoo hauv qab no tuaj yeem tsim los ntawm cov pH thiab cov pOH.
- Yeej, pH qhov tseem ceeb muab qhov tsis zoo logarithm ntawm hydrogen ion concentration, thaum tus nqi pOH muab qhov tsis zoo logarithm ntawm hydroxide ion concentration.
Qhov sib txawv ntawm qhov ntsuas pH thiab pOH tus nqi
Qhov sib txawv ntawm tus nqi ph thiab pOH tus nqi
Tom qab ntawd, peb muab koj cov yeeb yaj kiab uas koj tuaj yeem pom tias pH ntsuas qhov concentration ntawm hydrogen ions, thaum pOH ntsuas qhov concentrations ntawm hydroxyl anions lossis hydroxide ions.


