Fihirisar abubuwan da ke cikin shafi
En Ok Pool Reform, a cikin wannan sashe a cikin PH matakin wuraren iyo za mu bi da Me yasa pH na ruwan tafkin yake da mahimmanci?
Me yasa pH na ruwan tafkin yake da mahimmanci?
Menene matakin pH na tafkin da yadda ake sarrafa shi
Menene pH na tafkin don?
Ƙayyadaddun pH yana ɗaya daga cikin mafi mahimmanci kuma mafi yawan hanyoyin bincike da aka yi amfani da su a cikin ilmin sunadarai da biochemistry. pH yana ƙayyade abubuwa masu ban mamaki da yawa na tsari da ayyukan kwayoyin halitta, don haka halin sel da kwayoyin halitta.
Ingancin ruwan da ke cikin tafkin ya dogara kai tsaye akan pH, idan ba a kiyaye shi ba zai iya haifar da cututtuka.
pH yana da alaƙa ta kusa da ingancin ruwa a wuraren iyo. Wannan shi ne saboda chlorine kawai yana da tasiri idan pH na ruwan tafkin yana tsakanin 6.5 da 8. Idan pH na ruwa ya fi 8 ko ƙasa da 6.5, komai yawan chlorine da aka kara, ba zai yi aiki ba. Saboda wannan dalili, yana da mahimmanci don tabbatar da cewa pH yana tsakanin 6.5 da 8. Wannan tanadi yana da mahimmanci don tabbatar da cewa tafkin ya kasance a cikin kyakkyawan yanayi. Yawan pH na ruwa (fiye da 8) yana haifar da ruwan gajimare, ƙwanƙwasa da haushin idanu, kunnuwa, hanci da makogwaro.
Me yasa yake da mahimmanci a sarrafa pH na wuraren waha?

Tsabtace wuraren waha wani abu ne mai mahimmanci, da kuma kula da pH na yau da kullum wanda ke hana bayyanar ƙwayoyin cuta da kuma tabbatar da lafiyar masu wanka.s.
Yin amfani da sinadarai a cikin wuraren wanka yana da mahimmanci ga dalilai da yawa, daga cikinsu, lafiyar ruwa da kuma guje wa bayyanar ƙwayoyin cuta da ƙananan ƙwayoyin cuta. Duk da haka, waɗannan abubuwan da aka kara za su iya rashin daidaituwar pH na ruwa, haifar da haɗari ga lafiyar ɗan adam. A saboda wannan dalili, dole ne a fahimci mahimmancin daidaita wannan alamar, wane irin rawar da sauran abubuwa ke takawa, kamar caustic soda don wuraren waha da kuma wadanne dabarun aiwatarwa don bin matakan tsaro a cikin ayyukan wadannan wurare.
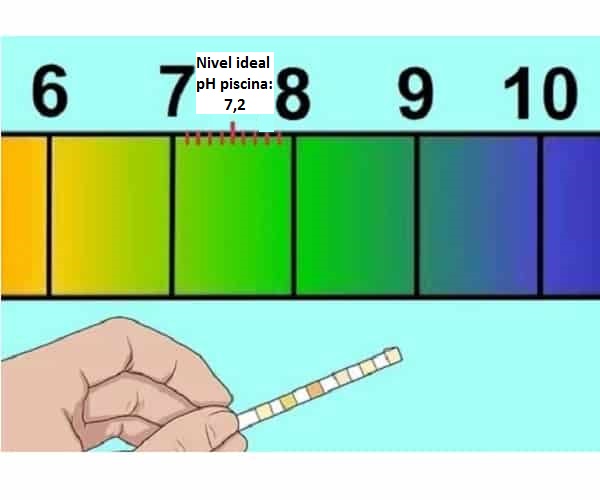
pH shine ma'aunin alkalinity (acidity) na ruwa wanda, gabaɗaya, yakamata ya kasance tsakanin 7,2 da 7,6. Dole ne a auna wannan alamar tare da ma'aunin chlorine, tun da dacewar ruwa don wanka ya dogara da waɗannan abubuwa biyu. Ga masana da yawa, madaidaicin ƙimar chlorine a cikin ruwa shine 1 ppm (sassan kowace miliyan) kuma don alkalinity 125 ko 150 ppm.
Ana ɗaukar pH na wurin wanka lokacin da ƙasa da 7,2 da alkaline lokacin da ya fi 7,6. Abubuwan da ke haifar da lafiyar mai yin ninkaya a cikin tafkin mai acidic pH sune ƙaiƙayi da ƙonewa a idanu, hanci da fata, haushin makogwaro, bayyanar eczema, bushewa, kururuwa, haushi, da sauransu.

Bugu da ƙari, acidity na ruwa na iya yin tasiri kai tsaye ga lalacewa da tsagewar wuraren tafki, musamman a kan waɗannan ƙarfe ko sassan siminti waɗanda za su iya lalata ta hanyar yawan acidity.
Sakamakon samun pH sama da 7,6 zai zama mafi mahimmanci a cikin ruwa fiye da lafiyar masu wanka. Ko da yake mutanen da suke wanka a cikin tafkin alkaline suna da alamun bayyanar cututtuka irin su bushewar fata da bushewa, babbar matsalar ita ce rashin kyawun yanayin ruwa, wanda zai zama gajimare, kore, yana da wari mara kyau kuma yana sauƙaƙa bayyanar ƙwayoyin cuta, ƙwayoyin cuta da ƙwayoyin cuta. algae . Ta wannan hanyar, ko da an ƙara matakin chlorine don kashe shi, babu abin da zai samu idan ba a daidaita pH ba.
Daidaita darajar pH a cikin jikin mutum
PH matakin

Menene pH na ruwan tafkin
Menene ma'anar PH pool?

Menene ma'anar pH na tafkin?
pH na tafkin wato
Menene pH na tafkin: pH shine yuwuwar hydrogen, ƙimar da ta dace da tattarawar ions hydrogen a cikin ruwan tafkin ku kuma saboda haka shine ƙididdiga wanda ke nuna matakin acidity ko asalin ruwa. . Sabili da haka, pH ne ke kula da nuna ƙaddamar da ions H + a cikin ruwa, ƙayyade yanayin acidic ko asali.
Madaidaicin ƙimar pH
Ma'auni na pH yana tafiya daga 1 zuwa 14, tare da pH 7 shine mafita mai tsaka tsaki.
pH shine darajar da aka bayyana tare da ma'aunin logarithmic tsakanin ƙimar 0 da 14.
Don haka, don auna acidity na ruwa, kuma a yanayinmu na ruwan tafkin, sunadarai kuma yanzu za mu yi amfani da su. Ma'aunin pH wanda ya haɗa da ƙima daga 0 zuwa 14.
manufa pool pH
PH Pool: ɗaya daga cikin mahimman sigogi a cikin kula da tafkin.
Madaidaicin ƙimar pH na ruwa: tsakanin 7.2 da 7.6 manufa kewayon tsaka tsaki pH.

Don haka, Samun pH a cikin wannan kewayon ba wai kawai yana da kyau don samun ruwa a cikin yanayi mafi kyau bas tunda low ko babba pH yana rage tasirin disinfection, amma kuma manufa don fata da idanu masu wanka.
Saline PH

ph saline pools
- Hakika, da ph saline pool ya zo ya zama iri ɗaya da wuraren waha da aka yi wa chlorine tun lokacin da aka kiyaye pool amfani da gishiri kuma yana buƙatar saka idanu akai-akai pH na ruwa.
- Sabili da haka, pH na wuraren tafkunan gishiri ya kamata kuma su sami a pH yana tsakanin 7 da 7,6, matakin da ya dace shine tsakanin 7,2 da 7,4.
Alamar acidic, tsaka tsaki da alkaline pH
Muhimmancin pH a cikin kula da tafkin
Ƙarfin sarrafa pH a cikin kula da tafkin
Idan ya zo ga kula da tafkin, kiyaye sinadarai na ruwa a ma'auni yana da matukar muhimmanci. Kuma kamar yadda muka bayyana, matakin pH shine, ta hanyoyi da yawa, tushen wannan ma'auni. Duk da yake akwai abubuwa da yawa waɗanda zasu iya shafar matakin pH, tare da ɗan ƙaramin aiki da kulawa na yau da kullun, zaku iya tabbatar da cewa ruwan ku ya tsaya a cikin kewayon da ya dace don kayan aikin tafkin ku kuma cikakke ga baƙi.
Don haka, sarrafa pH aiki ne mai mahimmanci don haɓaka ingancin ruwa. kuma, saboda haka, don guje wa kashe kuɗi mara amfani ta hanyar haɓaka amfani da samfuran kulawa, da duk wani haɗari ga lafiyar ku.
Ruwa, wanda tsarinsa shine H2O (2 hydrogen atom don oxygen atom), dole ne a daidaita daidaitattun daidaito don ba da tabbacin yin wanka ba tare da damuwa ba kamar ido da kumburin mucosal, ko yaduwar algae.
Mataki na farko mai mahimmanci a cikin maganin ruwa: daidaitawar pH
Kamar yadda aka sani, akwai samfuran sinadarai da yawa waɗanda ake buƙata don lalata tafkin da garanti daidai maganin kiyaye ruwa, wanda ya fara ta hanyar daidaita PH zuwa manufa tsakanin 7,2 da 7,4.
Don aiwatar da daidaitaccen iko na waɗannan matakan, muna da jerin abubuwa kits da na'urorin haɗi don nazarin pH na ruwa.
Sanin pH na ruwan tafkin yana da matukar muhimmanci don lafiyarmu da kuma samun ruwan tafkin a daidai yanayin.
Me yasa yana da mahimmanci don sanin yadda ake auna pH

Muhimmancin sanin yadda ake auna pH
Tabbas, yanzu za mu jera ku yawancin haɗin gwiwa a cikin rayuwar yau da kullun wanda aka samo asali na kai tsaye tare da pH kuma a wannan yanayin yana da amfani don sanin yadda ake sarrafawa da auna pH da aka ba da wannan.
Me yasa yake da mahimmanci don koyon yadda ake auna pH

- Na farko, halayen sunadarai a cikin ruwa suna shafar acidity ko alkalinity na maganin. Wannan yana da mahimmanci ba kawai a cikin dakin gwaje-gwajen ilmin sunadarai ba, har ma a masana'antu, dafa abinci, magani da kuma a cikin wuraren shakatawa idan aka yi la'akari da amfani da wuraren wanka.
- M, Ana tsara pH a hankali a cikin ƙwayoyin ɗan adam da jini. Matsakaicin pH na al'ada na jini yana tsakanin 7,35 da 7,45. Bambancin ko da kashi goma na pH na iya zama m.
- Ƙasa pH yana da mahimmanci ga germination da girma na amfanin gona. Bugu da kari, ruwan acid da ke haifar da gurbacewar yanayi da na dan Adam na canza acidity na kasa da ruwa, yana matukar shafar halittu masu rai da sauran matakai.
- Don ƙare, a cikin dafa abinci, ana amfani da canje-canje a cikin pH gasa da sha.
Yadda ake auna ƙimar pH da nau'ikan mita
Yadda za a auna PH PH
Yadda za a lissafta pH

Ana ƙididdige ma'aunin pH ta hanyar logarithm mara kyau.
Ƙimar pH shine logarithmic
pH shi ne logarithm na maida hankali na H ions+, tare da canza alamar: Hakazalika, an ayyana pOH azaman logarithm na maida hankali na OH ions-, tare da canza alamar: Za a iya kafa dangantaka mai zuwa tsakanin pH da pOH. Farawa daga bayanin samfurin ionic na ruwa (Kw):
Logarithmic pH Formula
- Log pH Formula: Lissafi pH ta amfani da pH equation: pH = -log[H3O+].
Menene ma'anar cewa ƙimar pH shine logarithmic
Gaskiyar cewa pH shine logarithmic yana nufin cewa tsakanin kowace raka'a na sikelin akwai ma'auni na 10 bambanci.
- Don haka, wannan yana nufin cewa pH 5 shine sau 10 acidic fiye da pH 6, kuma pH 4 shine sau 100 fiye da pH 6.
Yadda za a lissafta pH tare da logarithms?
sikelin na Ana ƙididdige pH ta hanyar a logarithm korau. A logarithm korau kawai yana nuna sau nawa yakamata a raba lamba. Ma'auni na pH za a iya rubuta kamar haka: pH = -log[H3O+]. Wani lokaci ana rubuta lissafin kamar haka: pH = -logu[H+].
Dalilin haɓaka ƙimar ƙimar pH: An haɓaka sikelin pH, ɗaukar ruwa azaman ma'auni.

- Gaskiyar gwaji ce kawai mole 1 a cikin moles na ruwa 5,50,000,000 na ionizes zuwa H+ ɗaya da OH- ɗaya.
- Wannan daidai yake da gram ɗaya na ions hydrogen a cikin lita 10.000.000 na ruwa.
- Don haka lita daya na ruwa ya kunshi 1/10.000.000 (ko) 1/107 na gram H+. Don amfanin yau da kullun, adadi 'Potency' kawai aka yi amfani da shi, tare da alamar pH da aka sanya a gabansa.
PH kalkuleta
Sakamakon rashin samun ƙimar pH daidai
Rashin isasshen pH yana da illa ga lafiya
- Batu na farko da ba za mu iya mantawa da shi ba shi ne cewa rashin isasshen pH na ruwa na iya yin illa ga lafiyarmu.
- Hanya ce ta yin iyo cikin aminci a cikin tafki ba tare da jin daɗi a cikin idanu ba, yawancin waɗannan ana haifar da su ta wurin wuraren waha tare da babban pH, kodayake akwai yanayin yin imani cewa ƙonawa da sauran rashin jin daɗi a cikin idanu da fata shine sakamakon. na chlorine a cikin ruwa.
Rashin isassun ƙimar pH ba shi da lalata ruwa
- Dole ne ku kiyaye cewa: Idan ba tare da ma'auni na pH mai kyau ba, lalatawar ruwa zai zama maras kyau, ba zai yi kyau ba don amfani da maganin kashe kwayoyin cuta.
Me yasa pH a cikin ruwan tafkin ke sauka ko sama?
Yadda za a tada pH na tafkin da abin da zai faru idan matakin ya yi ƙasa
Sakamakon pH pool da babban pH haddasawa
Menene zai faru idan matakin pH yana sama da ƙimar da aka ba da shawarar?
Sakamakon babban pH pool: Menene zai faru idan pH na tafkin ya yi girma

- Da farko, sakamakon babban pH pool yana sa ruwa ya zama mai wahala don zagayawa da kyau kuma sau da yawa, matsala ce da ta taso ta amfani da wasu nau'ikan matattara ko na'urar dumama ruwa.
- Alamun da ke jikinmu busassun fata ne da kuma haushi.
- Hakazalika, ruwan gajimare yana canza pH na tafkin, wani lokaci ta hanyar amfani da isasshen adadin chlorine ko samfurin amfanin yau da kullun don lalata ruwan.
- Kamar dai wannan bai isa ba, babban pH zai ƙarfafa samuwar lemun tsami a cikin tafkin wanda zai ƙare da ruwa mai tsabta. Wadannan ma'adinan lemun tsami za su kasance a cikin bututu da sauran kayan aiki, wanda zai shafi kwanciyar hankali da aiki mai kyau. Hakanan za su manne a bango da benaye, suna canza kamanni da tsabtar tafkin.
A ƙasa, idan yana da sha'awar ku, muna ba ku hanyar haɗi zuwa shafi inda muke nazarin duk sakamakon babban pH a cikin wuraren waha da abubuwan da zasu iya haifar da su.