En Ok Pool Reform, a cikin wannan sashe a cikin PH matakin wuraren iyo Za mu magance tambaya mai zuwa: Menene acidic da asali pH ke nufi?
Fihirisar abubuwan da ke cikin shafi
Menene pH a cikin tafkin kuma ta yaya matakansa zasu kasance?

Menene ma'anar pH mai kyau don wuraren waha (7,2-7,4)
Acronym pH yana nufin yuwuwar hydrogen kuma ma'auni ne da ke nuna acidity ko tushen ruwa.
Don haka, pH yana nufin yuwuwar hydrogen, ƙimar da ta yi daidai da ɗimbin ions hydrogen a cikin ruwa a cikin tafkin ku kuma saboda haka ƙididdigewa ne wanda ke nuna matakin acidity ko asali na ruwa. Sabili da haka, pH ne ke kula da nuna ƙaddamar da ions H + a cikin ruwa, ƙayyade yanayin acidic ko asali.
Sikelin ƙimar pH na ruwan wanka

Wadanne dabi'u ne ma'aunin ma'aunin pH na ruwa ya haɗa?
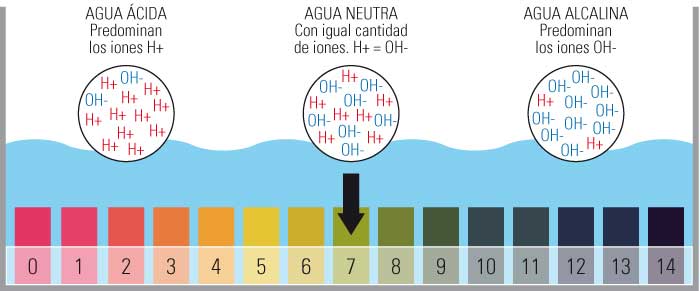
- Ma'aunin pH ya haɗa da ƙima daga 0 zuwa 14.
- Musamman kasancewa 0 mafi yawan acidic, 14 mafi asali da sanya pH mai tsaka tsaki a 7.
- Ana ƙayyade wannan ma'aunin ta adadin ions hydrogen kyauta (H+) a cikin abun.

Me yasa muke buƙatar pH?
pH wani ma'auni ne da ake amfani da shi don ƙididdige acidity ko asali na maganin ruwa. Ko maganin ruwa yana amsawa azaman acid ko tushe ya dogara da abun ciki na hydrogen ions (H+).
Duk da haka, ko da tsaftataccen ruwa mai tsafta da sinadari yana ɗauke da wasu ions hydrogen saboda raba ruwa da kai.
An sani cewa a ma'auni a ƙarƙashin daidaitattun yanayi (750 mmHg da 25 ° C), 1 L na ruwa mai tsabta ya ƙunshi. tawadar Allah
y
tawadar Allah
ions, saboda haka, ruwa a daidaitaccen zafin jiki da matsa lamba (STP) yana da pH na 7.
Abin da za a yi lokacin da pH na tafkin mu ba a tsara shi ba

Sanin sakamakon babban pH pool da kuma abubuwan da ke haifar da babban pH a cikin tafkin ku
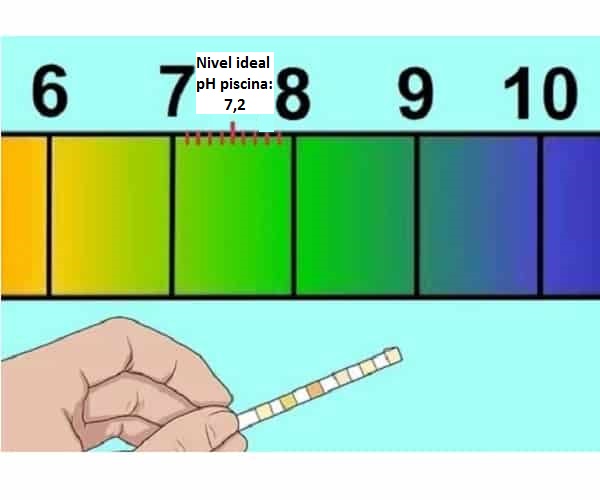
Yadda za a tada pH na tafkin da abin da zai faru idan ya yi ƙasa

Yadda za a Rage Babban ko Alkalin Pool pH
Jagora kan yadda ake yin gyare-gyaren tafkin ban da pH: tsaftace ruwa da lalata

Jagora mai amfani don sanin yadda ake tsaftace tafkin

Jagora don kula da tafki tare da ruwa a cikin kyakkyawan yanayin
Ta yaya pH na bayani zai iya zama?

pH na wani bayani
pH yana nufin "ikon hydrogen" ko "ikon hydrogen." pH shine mummunan tushen logarithm na 10 na aikin hydrogen ion.
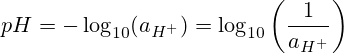
Duk da haka, a mafi yawan matsalolin sinadarai ba ma amfani da ayyukan ions hydrogen, amma ma'auni na molar ko molarity.

Ta yaya hanyoyin pH daban-daban suke
Don fara da, ya kamata ku san cewa pH sikelin logarithmic ne.
Sabili da haka, yana nufin cewa bambancin ta ɗaya yana nufin bambanci ta hanyar girma, ko sau goma kuma yana nuna yawan ions hydrogen a cikin maganin.
Don haka, ƙananan pH yana nuna babban taro na ions hydrogen kuma akasin haka.

Mene ne acid da tushe mahadi a cikin pH
Acids mai ƙarfi da tushe mai ƙarfi sune mahadi waɗanda, ga duk dalilai masu amfani, gaba ɗaya sun rabu cikin ions cikin ruwa.
Saboda haka za a iya la'akari da maida hankali na ions hydrogen a cikin irin waɗannan mafita daidai da ƙaddamarwar acid.
Lissafi na pH ya zama mai sauƙi
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Ƙididdigar pH ta amfani da ƙaddamarwar molar ya bambanta don ƙarfi acid / tushe da raunin acid / tushe.
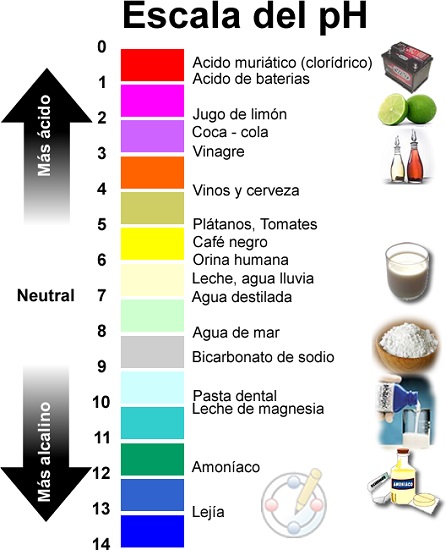
Alamar acidic, tsaka tsaki da alkaline pH
Rarraba Ma'aunin Ma'aunin pH

Menene ƙimar pH

Ma'auni na pH yana tafiya daga 1 zuwa 14, tare da pH 7 shine mafita mai tsaka tsaki.
Don haka, ya bayyana cewa pH shine darajar da aka bayyana tare da ma'auni na logarithmic tsakanin dabi'u 0 (matsakaicin acidic) da 14 (musamman alkaline); A tsakanin mu sami darajar 7 kasida a matsayin tsaka tsaki.
pH sikelin duniya pH nuna alama

Menene ma'anar cewa abu yana da matakin acidic ko alkaline pH?
Menene acid da tushe?
Acids da tushe abubuwa ne da ke wanzuwa a cikin yanayi kuma ana bambanta su ta matakin pH, wato, ta matakin acidity ko alkalinity. Ƙididdigar ko abubuwa na acidic ko alkaline ana sarrafa su ta hanyar matakin acidity ko alkalinity da aka auna ta hanyar ma'aunin pH kuma ya bambanta daga 0 (mafi girman acidic zuwa 14 (mafi girma alkaline). duk da haka suna da yawa masana'antu da aikace-aikace na mutum.
Yadda aka rarraba abubuwa bisa ma'auni na ƙimar pH
Rarraba abubuwa a cikin acid ko alkalines bisa ga ƙimar pH
Hakazalika, acidity da alkalinity sharuɗɗa biyu ne waɗanda ke amsa hanyar rarraba martanin kowane abu.

- Haka kuma, mun sake dagewa, Ma'auni na pH yana tafiya daga 1 zuwa 14, tare da pH 7 shine mafita mai tsaka tsaki.
- Idan pH bai wuce 7 ba, maganin shine acidic., yawan acid yana rage ƙimar pH don wannan dalili a acid shine wannan sinadari mai iya ba da gudummawar protons (H+) zuwa wani sinadari.
- A gefe guda, idan pH ya fi 7, ana kiran maganin asali (ko alkaline) kuma zai kasance mafi mahimmanci mafi girman pH; kuma kamar yadda aka nuna tushe shine sinadari mai iya kama protons (H+) na wani sinadari.
Menene alkaline ko asali bisa ga ma'aunin pH
Menene acidic abubuwa?
- Matsayin acid pH: pH kasa da 7
Menene ma'anar cewa ƙimar pH acidic?
- Cewa wani abu acidic yana nufin yana da wadatar H+ (hydrogen ions): pH fiye da 7
- Don haka, Acids abubuwa ne tare da pH ƙasa da 7. (pH na ruwa daidai da 7, wanda aka yi la'akari da tsaka tsaki), wanda ilimin sunadarai ya ƙunshi yawancin ions hydrogen lokacin ƙara ruwa. Yawancin lokaci suna amsawa da wasu abubuwa ta hanyar rasa protons (H+).
Menene tsaka tsaki abubuwa?
- Ƙimar pH mai tsaka tsaki: pH daidai da 7-
Menene ma'anar cewa ƙimar pH ta kasance tsaka tsaki?
- pH shine ma'auni na yadda acidic/ asali na ruwa yake.
- Kewayon yana daga 0 zuwa 14, tare da 7 kasancewa tsaka tsaki.
Menene abubuwan alkaline?
- Abubuwan da ke da tushe ko alkaline pH: pH fiye da 7.
Menene ma'anar cewa ƙimar pH shine alkaline?
- Cewa wani abu shine alkaline yana nufin cewa ba shi da talauci a cikin H+ (ko mai arziki a cikin tushen OH-, wanda ke kawar da H+).
- Duk wannan, Bases, a gefe guda, abubuwa ne tare da pH fiye da 7., wanda a cikin maganin ruwa yakan samar da ions hydroxyl (OH-) a tsakiya. Sun kasance suna zama masu ƙarfi oxidants, wato, suna amsawa da protons daga matsakaicin kewaye.
Menene acidity da alkalinity?
Menene acidity da alkalinity a cikin abinci
Bayan haka, a cikin bidiyon za a sanar da ku game da adadin abinci marasa iyaka da muke ci kowace rana amma,
- Shin kun taɓa mamakin dalilin da yasa wasu abubuwan dandano suke ɗaukar hankalinmu fiye da sauran?
- Abubuwan dandano kamar gishiri, burodi, abubuwan sha masu laushi, ruwan 'ya'yan itace, har da miya.
- Don menene wannan?
- Za mu bayyana muku duk wannan da ƙari a yanzu a cikin rikodin.
Theories na acidic da asali pH

Acid-base theories na pH
Menene Ka'idar Arrhenius pH?

Yaren mutanen Sweden ne suka gabatar da shi Svante Arrhenius ne a cikin 1884, ya ƙunshi ma'anar farko na zamani na acid da tushe a cikin sharuddan kwayoyin.
Arrhenius acid ph ka'idar
Abubuwan da ke rabuwa cikin ruwa don samar da cations hydrogen (H+).
Arrhenius asali pH ka'idar
Abubuwan da ke rabuwa cikin ruwa don samar da anions hydroxide (OH-).
KA'idar ARRHENIUS Menene acid? Menene tushe?
Arrhenius acid da ka'idar pH na asali
Brønsted-Lowry ph Theory
Menene ka'idar Brønsted-Lowry na pH?

An gabatar da shi a cikin 1923 da kansa ta Danish Johannes Nicolaus Bronsted da Ingilishi martin lowry, ya dogara ne akan ra'ayin conjugate acid-tushe nau'i-nau'i.
Lokacin da acid, HA, ya amsa tare da tushe, B, acid ɗin yana samar da tushen haɗin gwiwa, A.-, kuma tushe yana samar da acid conjugate, HB+, ta hanyar musayar proton (cation H+):
HA+B⇌A-+HB+
Brønsted-Lowry acid ph theory
Abu pH acid: mai iya ba da gudummawar protons (H+) ga tushen:
HA+H2O⇌A-+H3O+
Asalin ka'idar pH Brønsted-Lowry
Abubuwan da ke da pH na asali: mai iya karɓar protons (H+Acid:
B+H2O⇌HB++OH-
Ana ɗaukar wannan ka'idar a gama gari na ka'idar Arrhenius.
KA'idar BRÖNSTED-LOWRY Menene acid? Menene tushe?
Ka'idar pH bidiyo BRÖNSTED-LOWRY
Ma'anar aiki na yuwuwar ma'aunin pH

Menene ACIDITY da ALKALINITY?
Menene acidic da asali pH ke nufi?

acid pH
- Da farko, za mu iya samun bayani tare da acidic pH: wani abu da ya juya blue litmus takarda ja, reacts da wasu karafa, samar da gishiri da sakewa hydrogen (exothermic dauki).
- Bugu da ƙari, abubuwan da ke da pH acidic suna ba da rance tsakanin 0 da 7.
asali pH darajar

- Na biyu, akwai Base pH: Abubuwan da ke juya jajayen takarda litmus shuɗi kuma ya zama ruwan hoda lokacin da aka amsa da phenolphthalein.
- A gefe guda, nuna cewa suna da ƙimar pH tsakanin 7 da 14.
tsaka tsaki pH

- A ƙarshe, abu tare da ma'aunin pH mai tsaka tsaki shine wanda baya amsawa tare da alamomin tushen acid.
- Hakanan, pH na waɗannan abubuwa daidai yake da 7.
Abubuwan da ke da karfi acidic pH


Ma'auni na maganin acid a cikin pH
Yaya ƙimar acidic a cikin pH
- Acids suna sakin ions hydrogen, don haka maganin su na ruwa ya ƙunshi ƙarin ions hydrogen fiye da ruwa mai tsaka tsaki kuma ana ɗaukar acidic ƙasa da pH 7.
Menene mafi yawan samfuran pH mai ƙarfi acid
Akwai guda bakwai masu ƙarfi na gama gari:
- - hydrochloric acid HCl
- Nitric acid HNO3
- sulfuric acid H2SO4
- - hydrobromic acid HBr
- - HI hydroiodic acid
- - perchloric acid HClO4
- - chloric acid HClO3

Tsarin pH mai ƙarfi
m acid pH dabara
Tsarin pH mai ƙarfi: [HNO3] = [H3O+], da pH = -log[H3O+].
Yi lissafin ph kan layi mai ƙarfi acid
Yi lissafin pH na maganin acid mai ƙarfi.
Abubuwan da ke da ƙaƙƙarfan pH na asali

Ma'auni na mafita na asali a cikin pH

Yaya ƙimar acidic a cikin pH
Abubuwan halayen halayen tare da pH tushe
- Bases suna karɓar ions hydrogen (daure ga wasu ions hydrogen da aka kafa ta hanyar rarraba ruwa), don haka maganin su na ruwa ya ƙunshi ƙarancin ions hydrogen fiye da ruwa mai tsaka-tsaki kuma ana daukar su a sama da pH 7.

Formula don ƙididdige pH mai ƙarfi mai ƙarfi
m acid pH dabara
Tsarin pH mai ƙarfi: [HNO3] = [H3O+], da pH = -log[H3O+].
Menene mafi yawan samfuran pH mai ƙarfi acid
Har ila yau, babu tushe mai ƙarfi da yawa, kuma wasu daga cikinsu ba sa narkewa sosai a cikin ruwa. Wadanda suke mai narkewa su ne

- - sodium hydroxide NaOH
- - potassium hydroxide KOH
- - lithium hydroxide
- - rubidium hydroxide RbOH
- - Cesium hydroxide CsOH
Ƙarfi mai ƙarfi pH lissafi
Lissafi na pH mai ƙarfi
Abubuwa da dabaru tare da raunin acidic ko asali pH

Yaya ƙimar pH acid / rauni tushe
Babban halayyar raunin acid da tushe shine cewa an raba su cikin ruwa. An kafa ma'auni tsakanin matakai na gaba da baya, isa ga daidaiton yanayi wanda matakin rabuwa ya dogara da ƙarfin acid ko tushe.

Raunan acid / tushe kawai a wani yanki na rabuwa a cikin ruwa. Neman pH na acid mai rauni ya ɗan fi rikitarwa.

Tsarin pH mai rauni
tsarin pH mai rauni
Ma'aunin pH ya kasance iri ɗaya: , amma dole ne ku yi amfani da acid dissociation akai-akai (Ka) don nemo [H+].
Ma'anar Ka shine:
ina: - maida hankali na H+ ions
– maida hankali na conjugated tushe ions
– maida hankali na undissociated acid kwayoyin
don amsawa
Yi lissafin pH na maganin acid mai rauni.
Yi lissafin pH na maganin acid mai rauni.
Tsarin pH mai rauni
Formula don samun pH na tushe mai rauni
Yaya ake ƙididdige pH na tushe mai rauni?
Bayan samun pOH daga tsarin pOH na sama, da pH za ku iya ƙidaya amfani da dabara pH = pKw - pOH inda pK w = 14.00.
Bambance-bambance tsakanin menene ƙimar pH da pOH
Menene ƙimar pH ta al'ada?
- A wata hanya, pH shine ma'auni wanda ana amfani dashi don tabbatar da matakin acidity ko alkalinity na wani bayani. "p" yana nufin "mai yiwuwa", wanda shine dalilin da ya sa ake kiran pH: yuwuwar hydrogen.
Menene ƙimar pOH?
- A bangaren ku. pOH shine ma'auni na maida hankali na ions hydroxyl a cikin bayani. An bayyana shi azaman tushen logarithm mara kyau na 10 na maida hankali na hydroxyl ion kuma, sabanin pH, ana amfani dashi don auna matakin alkalinity na wani bayani.
Ƙididdige ƙarancin pH tushe
Lissafi na pH tushe mai rauni
Dangantakar Ƙarfin Acids da Bases

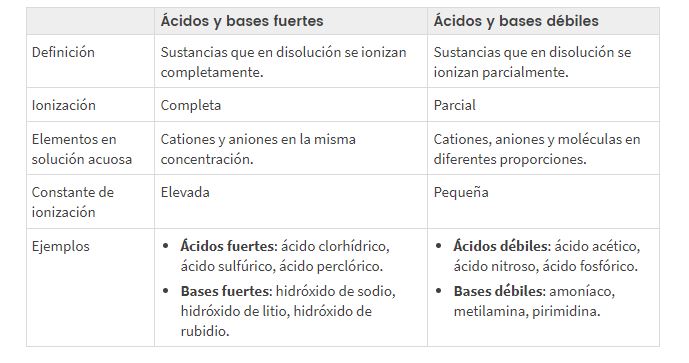
Bambanci tsakanin ƙarfi da rauni acidic da asali pH
Menene rarrabuwa mai ƙarfi da rauni acidic da asali pH ya dogara?
Dangane da yadda ionized ko rarraba acid ko tushe yake, muna bambanta tsakanin acid/bases mai ƙarfi da rauni, sharuddan da suka bayyana sauƙi para tuki la wutar lantarki (godiya ga mafi girma ko ƙarami kasancewar ions a cikin maganin).
KARFI DA RAUNAR ACIDS DA rarrabuwa, matakin rabuwa da Misalan pH
Rarraba pH mai rauni da ƙarfi acid da bastion
Matsayin ionization na acidic da asali pH
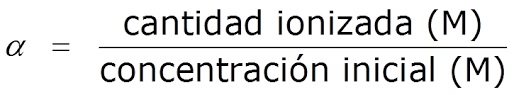
Menene matakin ionization ko rarraba acidic da asali pH
An kuma kira digiri na dissociation, α, an bayyana shi azaman rabo tsakanin adadin ionized acid/base da adadin farkon acid/base:
ááa=yawan acid/base/adadin acid/base na farko
Yawancin lokaci ana bayyana shi azaman kashi (%).
Menene matakin ionization ko rabuwar acidic da ainihin pH ke nufi?
acid mai karfi da tushe
Cikakken ionized (α≈1). Suna gudanar da wutar lantarki da kyau.
- Acid: HClO4, HI (aq), HBr (aq), HCl (aq), H2SO4 (1st ionization) da kuma HNO3.
- Bases: Hydroxides na alkali da alkaline ƙasa karafa.
Rauni acid da tushe
Ƙarƙashin ionized: α <1. Suna gudanar da wutar lantarki mara kyau.
- Acids: HF (aq), H2S (aq), H2CO3, H2SO3, H3PO4, H.N.O.2 da Organic acid, kamar CH3COOH.
- Bayani: NH3 (ya da NH4OH) da kuma tushen kwayoyin halitta na nitrogen, kamar amines.
Dissociation akai-akai pH acid da tushe
Menene rarrabuwa akai na asali da acidic pH?
Ma'auni ne na da karfi a acid/base a cikin bayani:
| ACID | BASE | |
|---|---|---|
| BALANCE | HA+H2O⇌A-+H3O+ | B+H2O⇌HB++OH- |
| M DUNIYA | Ka=[A-][H3O+][HA] | Kb=[HB+] [OH-] [B] |
| COLOGARHYTHM | pKa=-log Ka | pKb=-logKb |
Ƙarfin dangi na acidic da asali pH
Acid da asali pH akai-akai
Ion ma'auni na ruwa

Source: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Menene amphoteric
amphoteric menene su
A cikin ilmin sunadarai, wani abu mai amphoteric shine wanda zai iya amsawa azaman acid ko tushe.;
daga ina maganar ta fito amphoteric
Kalmar ta samo asali ne daga prefix na Hellenanci amphi- (αμφu-), ma'ana 'duka'. Yawancin karafa (kamar zinc, tin, gubar, aluminum, da beryllium) da yawancin metalloids suna da oxides ko hydroxide amphoteric.

Ruwa abu ne na amphiprotic
Me ake nufi da cewa Ruwa abu ne na amphiprotic
El ruwa abu ne amphiprotic (zai iya ba da gudummawa ko karɓar proton H+), wanda ke ba shi damar yin aiki azaman acid ko tushe (amphotericism).
Tsarin ma'auni na ionic ruwa

El ionic ma'auni na ruwa yana nufin halayen sinadarai wanda kwayoyin ruwa guda biyu ke amsawa don samar da ion oxonium (H3O+) da ion hydroxide (Oh-):
Ma'auni akai-akai, ake kira ionic samfurin ruwa, kuma KW ya nuna, ana iya ƙididdige shi ta samfurin:
Kw=[H3O+] [OH-]
25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH da samfurin ionic na ruwa (Kw). ACID-BASE
Alamar acid-base pH

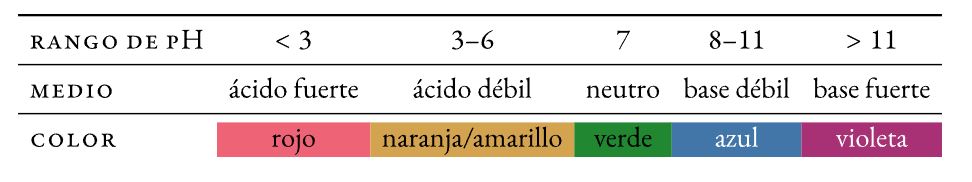
Un nuna alama pH shine mahaɗin sinadarai halochromic (yana canza launi -juya- kafin canje-canje a cikin pH) wanda aka ƙara a cikin ƙananan ƙididdiga zuwa bayani domin a gani na pH (acidity ko asali). Ana kiran canjin launi juya.
Litmus
Ruwa mai narkewar ruwa na rini daban-daban da aka ciro daga lichens. An sha kan takarda tace yana ɗaya daga cikin tsoffin alamun pH da aka yi amfani da su (~ 1300).

Methyl orange
Mai launi asali na azo wanda ya juya daga ja zuwa orange-yellow in matsakaiciyar acid:

Phenolphthalein
Alamar pH mara launi a matsakaicin acid wanda ke juya ruwan hoda a ciki matsakaici matsakaici:

duniya nuna alama
Mix na alamomi (thymol blue, methyl ja, bromothymol blue, da phenolphthalein) wanda ke nuna sauye-sauyen launi mai laushi akan nau'in pH mai fadi.
Acid-base neutralization titration

Acid-base titration/titration hanya ce ta bincike na kididdigar sinadarai
Menene hanyar nazarin sinadarai na acid da basci pH titration
Una acid-base titration/titration hanyar bincike ce ta ƙididdige sinadarai don tantance yawan adadin acid ko tushe da aka gano (nazari), neutralizing shi daidai tare da daidaitaccen bayani na tushe ko acid sanannen taro (m).

Titration/titration curve na 25 ml na 0.1 M acetic acid tare da 0.1 M sodium hydroxide.
Neutralization: amsawa tsakanin cakuda acid da tushe

Menene ya faru idan kun haɗu da acid da tushe?
Halin da ke tsakanin acid da tushe ana kiransa neutralization.
- Halayen tsaka-tsaki gabaɗaya exothermic ne. que ma'ana que Suna ba da kuzari a yanayin zafi.
- Se yakan kira su neutralization saboda lokacin da yake amsawa a acid tare da tushe,
- Saboda haka, abin da ke tsakanin acid da tushe ana kiransa neutralization. kuma fiye ko žasa yana kawar da abubuwan acidic ko na asali na mahadi guda biyu, wato suna kawar da kadarorin juna. samar da ruwa da gishiri maimakon.
Cakuda acid da tushe neutralizes kanta, pH ba dole ba ne ya zama tsaka tsaki.
- Dalilin da cewa cakuda acid da tushe neutralizes kanta pH ba dole ba ne ya zama tsaka tsaki yana dawwama saboda ta yawan adadin acid da/ko tushe ne aka ƙaddara pH a ƙarshe.
- Maimakon haka, Idan adadin H+ kuma OH- Haka ne, maganin ya zama tsaka tsaki saboda suna amsawa da juna don samar da ruwa (H+ +OH- →H20).
Dangane da yanayin acid da tushe mai amsawa, an bambanta lokuta huɗu:
- Da farko mai karfi acid + karfi tushe
- raunin acid + karfi tushe
- karfi acid + rauni tushe
- Kuma ƙarshe, raunin acid + raunin tushe
Menene acidic da asali pH neutralization dauki?
A cikin wani martani na neutralization, acid da tushe suna amsawa iri ɗaya irreversible don samar da gishiri da ruwa:
ACID + GASA ⟶ GISHIRI + RUWA
Dangane da ko titrant mai ƙarfi acid ne ko tushe, pH a daidai madaidaicin zai zama:
| ANALYTE/VALUANT | karfi/karfi | Rauni Acid/Karfafa Tushen | Gishiri mai rauni/Karfin Acid |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| NUNA (juyawa a tsakiya) | tsaka tsaki | Basic | Acid |
Yadda ake ƙididdige pH na Magani

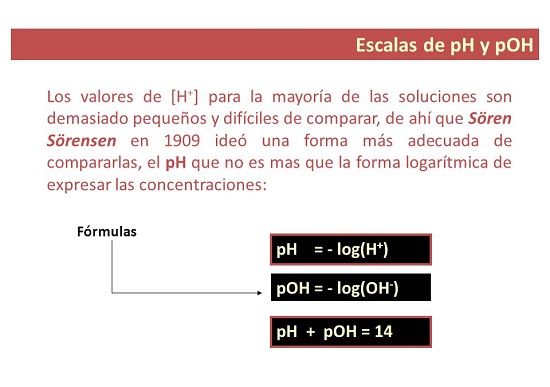
Menene ma'anar pH?
A cikin kimiyya, pH shine ma'aunin ions a cikin wani bayani. Kuna iya ƙididdige pH bisa ga taro.
Formula don ƙididdige pH
Yi lissafin pH ta amfani da ma'aunin pH: pH = -log[H3O+].
kalkuleta na pH don wuraren waha
Bidiyo yana lissafin pH na mafita
A cikin 1909, masanin kimiyyar halittu na Danish Soren Sorensen ya ba da shawarar kalmar pH don nuna "mai yiwuwa na hydrogen ion". Ya bayyana pH azaman logarithm na [H+] ya canza a cikin alamar. Sake bayyanawa azaman aikin [H3O+].
Magani pH Calculator

pH na Kalkuleta Magani
Yi lissafin pH na mafita
A ƙasa akwai ƙididdiga guda biyu waɗanda zaku iya amfani da su don bincika amsoshin matsalolin sinadarai.
- Na farko yana lissafin pH na maganin acid mai karfi o tushe mai karfi.
- Kuma, na biyu yana lissafin pH na maganin raunin acid o tushe mai rauni.
Yi lissafin pH na maganin acid/base mai ƙarfi
Kalkuleta don pH na maganin acid mai ƙarfi / tushe
[planetcalc cid=»8830″ harshe =»es» code =»» lakabin =»PLANETCALC, A pH na wani karfi acid/base bayani» launuka =»#263238,#435863,#090c0d,#fa7014,#fb9b5a,# c25004″ v=»4165″]
Yi lissafin pH na maganin acid/base mai rauni
Kalkuleta don pH na raunin acid/base bayani
[planetcalc cid =»8834″ harshe =»es» code =»» lakabin =»PLANETCALC, A pH na rauni acid/base bayani» launuka =»#263238,#435863,#090c0d,#fa7014,#fb9b5a,# c25004″ v=»4165″]