En Ok Pool Reform, muchikamu chino mukati me pH level madziva ekutuhwina Tichapindura mubvunzo unotevera: Chii chinonzi acidic uye yakakosha pH inorevei?
Index yezviri mukati pejiji
Chii chinonzi pH mudziva uye mazinga aro anofanira kunge ari sei?

Ko pH inorevei kumadziva ekushambira (7,2-7,4)
Acronym pH inomirira inogona hydrogen uye chiyero chinoratidza acidity kana basicity yemvura.
Saka, pH inoreva kugona kwehydrogen, kukosha kunoenderana nekusangana kwehydrogen ion mumvura iri mudziva rako uye saka ndiyo coefficient inoratidza chiyero cheasidhi kana kukosha kwemvura. Naizvozvo, iyo pH ndiyo inotarisira kuratidza huwandu hweH + ion mumvura, ichitarisa iyo acidic kana yakakosha hunhu.
Chiyero chepH kukosha kwemvura yekushambira yemvura
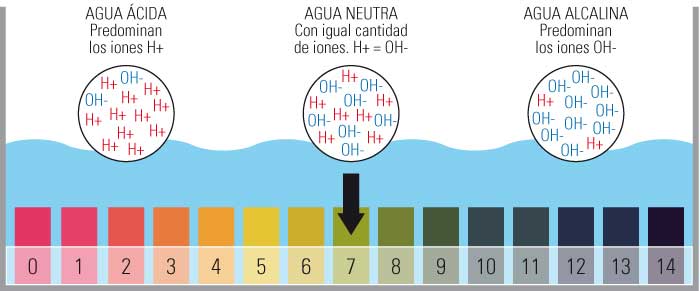

Ndezvipi zvakakosha izvo dziva remvura pH kuyerwa chiyero rinosanganisira?
- Iyo pH yekuyera chiyero inosanganisira kukosha kubva pa0 kusvika 14.
- Kunyanya kuve 0 yakanyanya acidic, 14 inonyanya kukosha uye nekuisa iyo Neutral pH pa7.
- Chiyero ichi chinotariswa nehuwandu hwemahara ayoni ehydrogen (H+) muchinhu.

Nei tichida pH?
pH chiyero chinoshandiswa kudoma acidity kana basicity yeaqueous solution. Kuti mhinduro ine aqueous inobata seasidhi kana chigadziko zvinoenderana nezviri mukati ma hydrogen ions (H+).
Nekudaro, kunyangwe mvura yemakemikari yakachena uye isina kwayakarerekera ine mamwe mahydrogen ions nekuda kwekuzviparadzanisa kwemvura.
Zvinozivikanwa kuti pakuenzanisa pasi pemamiriro akajairwa (750 mmHg uye 25°C), 1 L yemvura yakachena ine. at the mole
y
at the mole
ions, saka, mvura pachiyero tembiricha uye pressure (STP) ine pH ye7.
Zvekuita kana iyo pH yedziva redu ZVISINA kudzorwa

Ziva yakakwira pH dziva mhedzisiro uye zvikonzero zveyakakwira pH mudziva rako
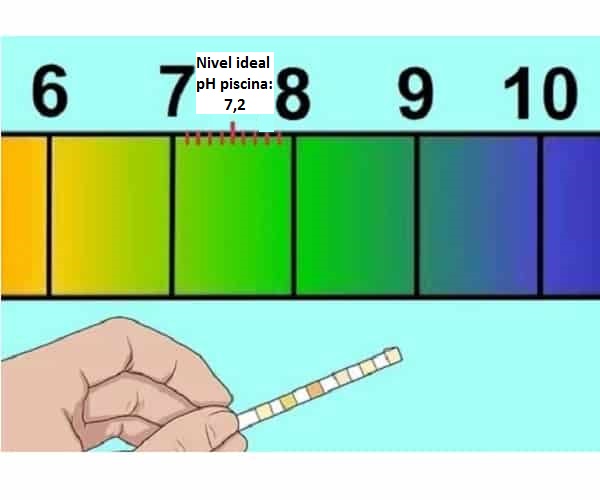
Nzira yekusimudza pH yedziva uye chii chinoitika kana yakaderera

Maitiro ekudzikisa Yepamusoro kana Alkaline Dziva pH
Nhungamiro dzemaitirwo ekugadzirisa dziva kuwedzera kune pH: kuchenesa mvura uye disinfection

Inobatsira gwara kuziva nzira yekuchenesa dziva

Nhungamiro yekuchengetedza dziva nemvura mune yakakwana mamiriro
Iyo pH yemhinduro inogona sei kuve?

pH yemhinduro
pH inomirira "hydrogen potential" kana "simba rehydrogen." Iyo pH ndiyo yakaipa ye base 10 logarithm yehydrogen ion chiitiko.
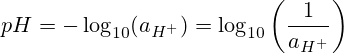
Nekudaro, mumatambudziko mazhinji emakemikari hatishandisi kuita kwehydrogen ions, asi iyo molar concentration kana molarity.

Ndeapi akasiyana pH mhinduro
Kutanga, iwe unofanirwa kuziva kuti iyo pH chiyero ndeye logarithmic.
Naizvozvo, zvinoreva kuti mutsauko neimwe nzira mutsauko nehurongwa hwehukuru, kana kagumi uye inversely inoratidza kusangana kwehydrogen ion mumhinduro.
Nokudaro, pH yakaderera inoratidza huwandu hwehuwandu hwehydrogen ions uye zvinopesana.

Chii chinonzi asidhi uye base macomputer mupH
Asidhi yakasimba uye mabhesi akasimba makomisheni ayo, kune ese anoshanda, anopatsanurwa zvachose mumaoni avo mumvura.
Nokudaro kuwanda kwehydrogen ions mumhinduro dzakadaro kunogona kuonekwa kuenzana nekusangana kweasidhi.
Kuverenga kwepH kunova nyore
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Kuverengera kwepH uchishandisa molar concentration kwakasiyana kune yakasimba asidhi/base uye isina kusimba asidhi/base.
Asidhi, kusarerekera uye alkaline pH kukosha
Kurongwa kweChiyero chepH Values

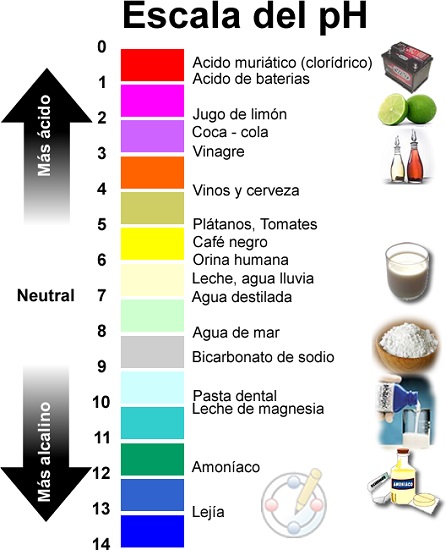
Chii chinonzi pH values

Chiyero chepH chinoenda kubva pa1 kusvika pa14, nepH 7 iri mhinduro isina kwayakarerekera.
Saka, zvinoitika kuti pH kukosha kunoratidzwa pachiyero che logarithmic pakati pehukoshi 0 (yakanyanya acidic) uye 14 (yakanyanya alkaline); Pakati tinowana kukosha 7 kwakanyorwa sekwakarerekera.
pH chiyero chepasi rose pH chiratidzo

Zvinorevei kuti chinhu chine acidic kana alkaline pH level?
Chii chinonzi acids uye mabhesi?
Acids uye mabhesi zvinhu zviripo muzvisikwa uye zvinosiyaniswa neiyo pH level, kureva, nedhigirii re acidity kana alkalinity. Maonerwo ekuti zvinhu zvine acidic here kana kuti alkaline zvinodzorwa nechiyero cheasidhi kana alkalinity inoyerwa kuburikidza neph scale uye inotangira pa0 (yakanyanya acidic kusvika pa14 (yakanyanya alkaline).Zvisinei, zvese zviri zviviri zvinhu zvinoparadza, kazhinji zvine chepfu, izvo zvisinei vane akawanda maindasitiri neanoshandiswa nevanhu.
Zvikamu zvinoiswa sei pachiyero chepH kukosha
Kurongwa kwezvinhu muasidhi kana alkaline zvichienderana nepH kukosha
Saizvozvo, acidity uye alkalinity mazwi maviri anopindura nzira yekuisa mumapoka maitiro echero chinhu.

- Saizvozvowo, tinosimbisa zvakare, Chiyero chepH chinoenda kubva pa1 kusvika pa14, nepH 7 iri mhinduro isina kwayakarerekera.
- Kana pH iri pasi pe7, mhinduro ine acidic., iyo acid yakawanda inodzika iyo pH kukosha kwechikonzero ichocho acid ndicho chinhu chemakemikari chinokwanisa kupa mapurotoni (H+) kune imwe kemikari.
- Panzvimbo iyoyo, kana pH yakakura kudarika 7, mhinduro inonzi basic (kana alkaline) uye ichave yakanyanya kukosha iyo yakakwirira pH yayo; uye sezvakaratidzwa hwaro ndicho chinhu chemakemikari chinokwanisa kubata mapurotoni (H+) yeimwe kemikari.
Chii chinonzi alkaline kana chakakosha zvinoenderana nechiyero chepH
Chii chinonzi acidic zvinhu?
- Acid pH level: pH isingasviki 7
Zvinorevei kuti pH kukosha ine acidic?
- Kuti chinhu chine acidic zvinoreva kuti chakapfuma muH+ (hydrogen ions): pH yakakura kupfuura 7
- Saka, Asidhi zvinhu zvine pH isingasviki 7. (pH yemvura yakaenzana ne7, inofungidzirwa kuti haina kwayakarerekera), iyo kemikari yacho inowanzova neayoni akawanda ehydrogen pakuwedzera mvura. Vanowanzo kuita nezvimwe zvinhu nekurasikirwa nemaproton (H+).
Ndezvipi zvinhu zvisina kwazvakarerekera?
- Neutral pH kukosha: pH yakaenzana ne7-
Zvinorevei kuti pH kukosha haina kwayakarerekera?
- pH chiyero chekuti mvura ine acidic sei/yakanyanya sei.
- Mutsara unobva pa0 kusvika pa14, uye 7 asina kwaakarerekera.
Chii chinonzi alkaline substances?
- Zvinhu zvine base kana alkaline pH: pH yakakura kupfuura 7.
Zvinorevei kana pH kukosha iri alkaline?
- Kuti chinhu chine alkaline zvinoreva kuti chakashata muH+ (kana kupfuma muOH mabhesi-, izvo zvinoita kuti H+).
- Kune izvi zvese, Mabhesi, kune rumwe rutivi, zvinhu zvine pH yakakura kupfuura 7., iyo mumhinduro dzine aqueous inowanzopa hydroxyl ions (OH-) pakati. Vanowanzova maoxidants ane simba, ndiko kuti, vanobatana nemaproton kubva kune yakatenderedza svikiro.
Chii chinonzi acidity uye alkalinity?
Chii chinonzi acidity uye alkalinity muchikafu
Zvadaro, muvhidhiyo iwe uchaziviswa nezvehuwandu husingagumi hwechikafu chatinodya zuva nezuva asi,
- Wakambozvibvunza here kuti nei mamwe maflavour achibata pfungwa dzedu kupfuura mamwe?
- Kunhuhwirira kwakadai semunyu, chingwa, zvinwiwa, muto, kunyange sosi.
- Chii ichi?
- Isu tichakutsanangurira zvese izvi nezvimwe zvakawanda mukurekodha.
Dzidziso dze acidic uye yakakosha pH

Acid-base theories yepH
Chii chinonzi Arrhenius pH Theory?

yakakurudzirwa neSweden Svante Arrhenius muna 1884, inoumba tsananguro yekutanga yemazuva ano yeasidhi uye mabhesi mumashoko emamorekuru.
Arrhenius acid ph theory
Chinhu chinopatsanura mumvura kuita hydrogen cations (H+).
Arrhenius basic pH theory
Chinhu chinoparadzanisa mumvura kuita hydroxide anions (OH-).
ARRHENIUS THEORY Chii chinonzi asidhi? Chii chinonzi nheyo?
Arrhenius acid uye basic pH theory vhidhiyo
Brønsted-Lowry ph theory
Chii chinonzi Brønsted-Lowry theory yepH?

Yakakurudzirwa muna 1923 yakazvimirira neDanish Johannes Nicolaus Bronsted uye Chirungu Martin lowry, inobva papfungwa ye conjugate acid-base pairs.
Kana asidhi, HA, ikaita chigadziko, B, asidhi inoumba hwaro hwayo, A.-, uye hwaro hunoumba conjugate acid yayo, HB+, nekuchinjanisa proton (cation H+):
HA+B⇌A−+HB+
Brønsted-Lowry acid ph theory
Substance pH acid: inokwanisa kupa mapurotoni (H+) kune chikonzero:
HA+H2O⇌A−+H3O+
Basic pH theory Brønsted-Lowry
Chinhu chine pH yakakosha: inokwanisa kugamuchira mapurotoni (H+) yeasidhi:
B+H2O⇌HB++OH−
Dzidziso iyi inonzi a generalization yedzidziso ye Arrhenius.
BRÖNSTED-LOWRY THEORY Chii chinonzi asidhi? Chii chinonzi nheyo?
pH theory vhidhiyo BRÖNSTED-LOWRY
Tsanangudzo dzekushanda dzezviyero zvinogoneka zvepH

Chii chinonzi ACIDITY uye ALKALINITY?
Chii chinonzi acidic uye yakakosha pH inorevei?

acid pH
- Chekutanga, tinogona kuwana mhinduro ine acidic pH: chinhu chinoshandura bhuruu litmus bepa tsvuku, inopindirana nedzimwe simbi, ichigadzira munyu uye kuburitsa hydrogen (exothermic reaction).
- Pamusoro pezvo, zvinhu zvine acidic pH zvinokweretesa kukosha pakati pe0 ne7.
yakakosha pH kukosha

- Chechipiri, pane Base pH: Chinhu chinoshandura red litmus bepa bhuruu uye chinoshanduka pink kana chaitwa ne phenolphthalein.
- Kune rumwe rutivi, ratidza kuti vane pH kukosha pakati pe7 ne14.
kwazvakarerekera pH

- Chekupedzisira, chinhu chine kwazvakarerekera pH kuyerwa ndechiya chisingaite ne acid-base zviratidzo.
- Zvakare, iyo pH yezvinhu izvi yakaenzana ne7.
Zvinhu zvine acidic yakasimba pH


Kuyera kweasidhi mhinduro mupH
Ndeipi iyo acidic kukosha mupH
- Acids inoburitsa ma hydrogen ions, saka mhinduro dzawo dzine mvura ine ma hydrogen ions akawanda kupfuura mvura isina kwayakarerekera uye inoonekwa se acidic pazasi pH 7.
Ndeapi anonyanya kusimba asidhi pH zvigadzirwa
Kune manomwe chete akajairika akasimba asidhi:
- - hydrochloric acid HCl
- - nitric asidhi HNO3
- - sulfuric acid H2SO4
- - hydrobromic acid HBr
- - HI hydroiodic acid
- - perchloric acid HClO4
- - chloric acid HClO3

Yakasimba acid pH formula
yakasimba acid pH formula
Asidhi yakasimba pH formula: [HNO3] = [H3O+], uye pH = -log[H3O+].
Verenga ph online yakasimba asidhi
Verenga pH yemhinduro yakasimba yeasidhi.
Zvinhu zvine hwakasimba hwakasimba pH

Kuyerwa kwezviyero zvekutanga mupH

Ndeipi iyo acidic kukosha mupH
Hunhu zvinhu zvine hwaro pH
- Mabhesi anogashira maion ehydrogen (anosunga kune mamwe maioni ehydrogen akaumbwa nekuparadzaniswa kwemvura), saka mhinduro dzawo dzine mvura dzine maion ehydrogen mashoma pane mvura isina kwayakarerekera uye inoonekwa seyakakosha pamusoro pepH 7.

Formula yekuverenga yakasimba basic pH
yakasimba acid pH formula
Asidhi yakasimba pH formula: [HNO3] = [H3O+], uye pH = -log[H3O+].
Ndeapi anonyanya kusimba asidhi pH zvigadzirwa
Iko hakunawo mabhesi akawanda akasimba, uye mamwe acho haana kunyanya kunyungudika mumvura. Izvo zvinonyungudika ndizvo

- - sodium hydroxide NaOH
- - potassium hydroxide KOH
- - lithium hydroxide LiOH
- - rubidium hydroxide RbOH
- - cesium hydroxide CsOH
Yakasimba base pH kuverenga
Kuverengera kwehwaro hwakasimba pH
Zvinhu uye mafomula ane isina kusimba acidic kana yakakosha pH

Ko pH inokoshesa sei acid / isina simba base
Hunhu hukuru hweasina simba acid uye mabhesi ndeokuti akapatsanurwa zvishoma mumvura. Kuenzana kunotangwa pakati penzira dzemberi nedzokudzokera shure, kusvika padanho rakadzikama umo dhigirii rekuparadzanisa rinoenderana nesimba reasidhi kana chigadziko.

Maasidhi asina simba/mabhesi anongopatsanurwa zvishoma mumvura. Kuwana pH yeasidhi isina simba kunowedzera kuoma.

Weak Acid pH Formula
isina simba acid pH formula
Iyo pH equation inoramba yakafanana: , asi iwe unofanirwa kushandisa iyo acid dissociation nguva dzose (Ka) kutsvaga [H+].
Formula yeKa inoti:
kupi: - kusanganiswa kweH + ions
- kusangana kweiyo conjugated base ions
- kusanganiswa kweasina kusanganiswa asidhi mamorekuru
nokuda kwekuita
Verenga pH yeiyo isina kusimba asidhi mhinduro.
Verenga pH yeiyo isina kusimba asidhi mhinduro.
Yakashata base pH formula
Formula yekuwana pH yehwaro husina kusimba
Ko iyo pH yehwaro husina kusimba inoverengerwa sei?
Mushure mekuwana pOH kubva pane iri pamusoro pOH formula, iyo pH iwe unogona kuverenga kushandisa formula pH =pKw - pOH apo pK w = 14.00.
Misiyano pakati peiyo kukosha kwepH uye pOH
Chii chinonzi pH kukosha?
- Neimwe nzira, pH chiyero icho inoshandiswa kumisa mwero we acidity kana alkalinity yemhinduro. Izwi rekuti “p” rinomirira “kugona”, ndosaka pH ichinzi: kugona kwehydrogen.
Chii chinonzi pOH kukosha?
- Nekuda kwako. pOH chiyero chehuwandu hwehydroxyl ions mumushonga. Inoratidzwa sehwaro 10 negative logarithm yehydroxyl ion concentration uye, kusiyana nepH, inoshandiswa kuyera alkalinity level yemhinduro.
Verenga isina simba base pH
Kuverenga kweiyo isina simba base pH
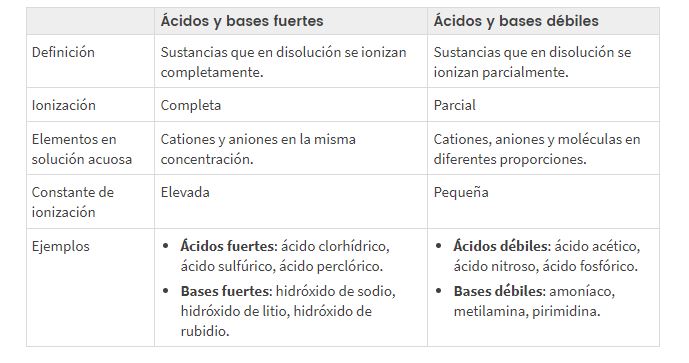
Relative Kusimba kweAcids uye Mabhesi

Musiyano pakati peakasimba uye asina kusimba acidic uye yakakosha pH
Ko kupatsanurwa kweiyo yakasimba uye isina kusimba acidic uye yakakosha pH inoenderana nei?
Zvichienderana nekuti ionized kana kuparadzaniswa sei asidhi kana hwaro, tinosiyanisa pakati yakasimba uye isina simba asidhi/mabhesi, mashoko anotsanangura the nzvimbo nokuti kutyaira la magetsi (nekuda kwekuvapo kukuru kana kushoma kweion mune mhinduro).
ASIMBA UYE WEAK ACIDS UYE BASES kupatsanura, dhigirii rekuparadzanisa uye pH Mienzaniso
Classification pH isina simba uye yakasimba asidhi uye bastion
Dhigirii yeionization yeasidhi uye yakakosha pH
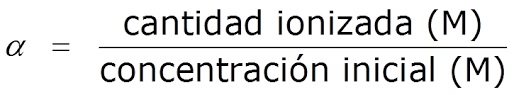
Ndeipi dhigirii yeionization kana dissociation yeasidhi uye yakakosha pH
Zvakare inonzi degree of dissociation, α, inotsanangurwa sereshiyo pakati pehuwandu hweionized asidhi / base uye huwandu hwekutanga asidhi / chigadziko:
áá= kuwanda kweasidhi ionized/base/huwandu hwekutanga asidhi/chigadziko
Inowanzotaridzwa sechikamu (%).
Ko dhigirii reionization kana dissociation yeasidhi uye yakakosha pH inorevei?
asidhi yakasimba uye mabhesi
Yakazara ionized (α≈1). Vanofambisa magetsi zvakanaka.
- Acids: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (1st ionization) uye HNO3.
- Mabhesi: Hydroxides yealkali uye alkaline pasi simbi.
Maasidhi asina simba uye mabhesi
Muchidimbu ionized: α <1. Vanoshandisa magetsi zvisina kunaka.
- Acids: HF(aq), H2S(aq), H2CO3, H2SO3, H3PO4, H.N.O.2 uye organic acids, senge CH3COOH.
- Nheyo: NH3 (kana NH4OH) uye nitrogenous organic base, senge mamines.
Dissociation inogara pH acids uye mabhesi
Chii chinonzi dissociation chenguva dzose cheyakakosha uye acidic pH?
Icho chiyero che simba a acid/base mumhinduro:
| ACID | BASE | |
|---|---|---|
| BALANCE | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| CONSTANT | Ka=[A−][H3O+][HA] | KB=[HB+][OH−][B] |
| COLOGARHYTHM | pKa=−logKa | pKb=−logKb |
Simba rehukama hwe acidic uye yakakosha pH
Acid uye yakakosha pH nguva dzose
Ion chiyero chemvura

mabviro: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Chii chinonzi amphoteric
amphoteric chii ivo
Mumakemikari, chinhu chinonzi amphoteric ndicho chinogona kuita seasidhi kana chigadziko..
shoko rinobvepi amphoteric
Izwi rinobva pashoko rekutanga rechiGiriki rokuti amphi- (αμφu-), rinoreva 'zvose'. Masimbi akawanda (sezinc, tin, lead, aluminium, uye beryllium) uye mametalloid mazhinji ane. oxides kana hydroxides amphoteric.

Mvura chinhu chinonzi amphiprotic
Zvinorevei kuti Mvura is amphiprotic substance
El mvura chinhu amphiprotic (inogona kupa kana kugamuchira proton H+), iyo inobvumira kuti iite seasidhi kana chigadziko (amphotericism).
Mvura ionic balance formula

El ionic chiyero chemvura inoreva kuita kwemakemikari umo mamorekuru maviri emvura anoita kuburitsa ayoni oxonium (H3O+) uye ion hydroxide (oh-):
The equilibrium constant, inonzi ionic chigadzirwa chemvura, uye inoratidzwa naKw, inogona kufananidzwa nechigadzirwa:
Kw=[H3O+][OH−]
Pa25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH uye ionic chigadzirwa chemvura (Kw). ACID-BASE
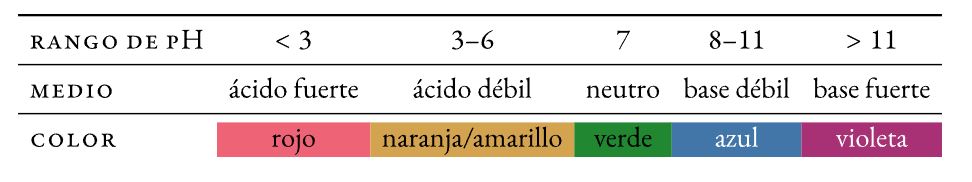
Acid-base pH zviratidzo

Un chiratidzo pH ikemikari inosanganiswa halochromic (inoshandura ruvara rwayo -bend- isati yachinja mupH) iyo inowedzerwa mune zvidiki kune mhinduro kuti ione nekuona pH yayo (acidity kana basicity). Kuchinja kwemavara kunonzi tendeuka.
Litmus
Musanganiswa wemvura unonyungudika wedhayi dzakasiyana dzakatorwa kubva lichen. Yakanyudzwa papepa resefa, inoumba imwe yekare pH zviratidzo zvakashandiswa (∼ 1300).

Methyl orange
Colourant azo derivative inoshanduka kubva kutsvuku kuenda kuorenji-yero mukati acid yepakati:

Phenolphthalein
isina ruvara pH chiratidzo mune acid yepakati inoshandura pink mukati basic medium:

chiratidzo chepasi rose
Musanganiswa wezviratidzo (thymol bhuruu, methyl tsvuku, bromothymol bhuruu, uye phenolphthalein) iyo inoratidza hunyoro shanduko yemavara pamusoro pehupamhi hwepH kukosha.
Acid-base neutralization titrations

Acid-base titration/titration inzira yehuwandu hwekuongorora kemikari
Chii chinonzi acid uye basci pH titration kemikari yekuongorora nzira
Una acid-base titration/titration inzira yehuwandu hwekuongorora makemikari nzira yekuona kusangana kweasidhi yakaonekwa kana chigadziko (analyte), kuigadzirisa chaizvo neyakajairwa mhinduro yechigadziko kana acid yeinozivikanwa yekumisikidzwa (mhare).

Titration/titration curve ye25 mL ye0.1 M acetic acid ine 0.1 M sodium hydroxide.
Neutralization: kuita pakati pemusanganiswa weasidhi uye hwaro

Chii chinoitika kana ukasanganisa asidhi nechigadziko?
Kuita pakati peasidhi nechigadziko kunonzi neutralization.
- Neutralization maitiro anowanzo exothermic. , que zvinoreva , que Vanopa simba nenzira yekupisa.
- Se kazhinji anodzidaidza kuti neutralization nekuti pakuita a acid na a hwaro,
- Naizvozvo, kuita pakati peasidhi nemabhesi kunonzi neutralization. uye zvakanyanya kana zvishoma zvinobvisa iyo acidic kana yakakosha zvimiro zvezvese zviri zviviri makomisheni, ndiko kuti, ivo vanoderedza hunhu hweumwe neumwe. kubudisa mvura nemunyu pachinzvimbo.
Musanganiswa weasidhi uye chigadziko chinozvimisa pachako, iyo pH haifanirwe kuve isina kwayakarerekera.
- Chikonzero chekuti musanganiswa weasidhi uye chigadziko chinozvimisa iyo pH haifanire kuve isina kwayakarerekera inotsigirwa nekuti ihuwandu hweasidhi uye / kana chigadziko icho pH inozotemerwa.
- Pane kudaro, Kana huwandu hweH+ uye oh- zvakafanana, mhinduro inova isina kwayakarerekera nekuti ivo vanobatana kuti vaumbe mvura (H+ +OH- →H20).
Zvinoenderana nehunhu hweasidhi uye hwaro hwekuita, nyaya ina dzinosiyaniswa:
- Pakutanga asidhi yakasimba + hwaro hwakasimba
- asidhi isina simba + hwaro hwakasimba
- yakasimba asidhi + isina simba base
- Uye chekupedzisira, isina simba asidhi + isina simba base
Chii chinonzi acidic uye chakakosha pH neutralization reaction?
Mukuita kwe neutralization, asidhi uye chigadziko chinoita nenzira imwecheteyo zvisingachinjiki kugadzira munyu nemvura:
ACID + BASE ⟶ MUNYU + MVURA
Zvichienderana nekuti iyo titrant iasidhi yakasimba kana chigadziko, iyo pH pane yakaenzana poindi ichave:
| ANALYTE/VALUTAN | akasimba/akasimba | Isina Simba Asidhi / Yakasimba Base | Yakaneta Base / Yakasimba Asidhi |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| INDICATOR (inotenderera nepakati) | Kusarerekera | Basic | Acid |
Maitiro ekuverengera pH ye Solution

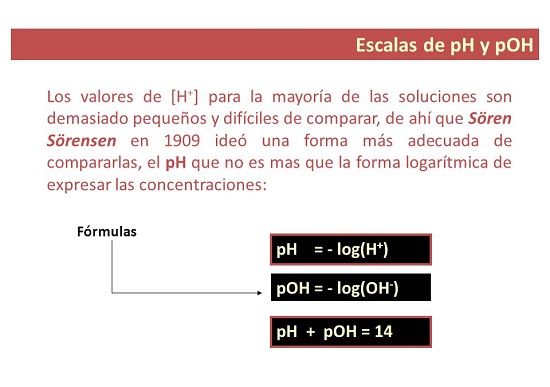
Chii chinonzi pH formula?
Musainzi, pH ndiyo chiyero cheiyoni mumhinduro. Iwe unogona kuverengera pH zvichibva pane iyo concentration.
Formula yekuverenga pH
Verenga pH uchishandisa pH equation: pH = -log[H3O+].
pH Calculator yemadziva ekushambira
Vhidhiyo inoverengera pH yemhinduro
Muna 1909, Danish biochemist Soren Sorensen akakurudzira izwi rekuti pH kuratidza "inogoneka yehydrogen ion". Akatsanangura pH serogarithm ye [H+] yachinja muchiratidzo. Kutsanangura patsva sebasa re [H3O+].
Solution pH Calculator

Iyo pH yeSolution Calculator
Verenga pH yemhinduro
Pazasi pane macalculator maviri aungashandisa kutarisa mhinduro kumatambudziko ekemesitiri.
- Yekutanga inoverengera iyo pH yemhinduro ye asidhi yakasimba o hwaro hwakasimba.
- Uye, yechipiri inoverenga iyo pH yemhinduro ye asidhi isina simba o weak base.
Verenga pH yeasidhi yakasimba / base solution
Calculator ye pH yeasidhi yakasimba/ base solution
[planetcalc cid=»8830″ mutauro=»es» code=»» label=»PLANETCALC, Iyo pH yeakasimba acid/base solution» mavara=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]
Verenga pH yeasidhi isina simba / base solution
Calculator ye pH yeasidhi isina simba/ base solution
[planetcalc cid=»8834″ mutauro=»es» kodhi=»» label=»PLANETCALC, Iyo pH yeasidhi isina simba/base solution» mavara=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]