
Index yezviri mukati pejiji
En Ok Pool Reform, muchikamu chino mukati me pH level madziva ekutuhwina tichabata the musiyano pakati pe ph uye poh mumadziva emvura kukosha.
Chii chinonzi pH mudziva uye mazinga aro anofanira kunge ari sei?

Ko pH inorevei kumadziva ekushambira (7,2-7,4)
Acronym pH inomirira inogona hydrogen uye chiyero chinoratidza acidity kana basicity yemvura.
Saka, pH inoreva kugona kwehydrogen, kukosha kunoenderana nekusangana kwehydrogen ion mumvura iri mudziva rako uye saka ndiyo coefficient inoratidza chiyero cheasidhi kana kukosha kwemvura. Naizvozvo, iyo pH ndiyo inotarisira kuratidza huwandu hweH + ion mumvura, ichitarisa iyo acidic kana yakakosha hunhu.
Chiyero chepH kukosha kwemvura yekushambira yemvura
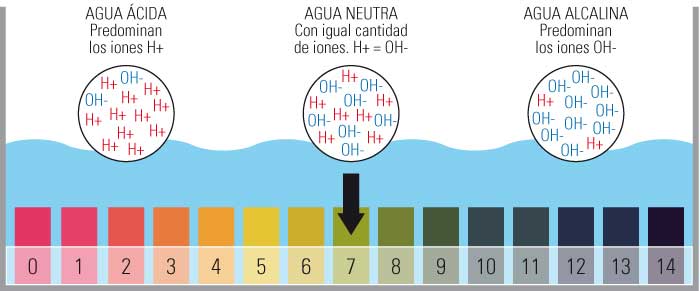

Ndezvipi zvakakosha izvo dziva remvura pH kuyerwa chiyero rinosanganisira?
- Iyo pH yekuyera chiyero inosanganisira kukosha kubva pa0 kusvika 14.
- Kunyanya kuve 0 yakanyanya acidic, 14 inonyanya kukosha uye nekuisa iyo Neutral pH pa7.
- Chiyero ichi chinotariswa nehuwandu hwemahara ayoni ehydrogen (H+) muchinhu.

Nei tichida pH?
pH chiyero chinoshandiswa kudoma acidity kana basicity yeaqueous solution. Kuti mhinduro ine aqueous inobata seasidhi kana chigadziko zvinoenderana nezviri mukati ma hydrogen ions (H+).
Nekudaro, kunyangwe mvura yemakemikari yakachena uye isina kwayakarerekera ine mamwe mahydrogen ions nekuda kwekuzviparadzanisa kwemvura.
Zvinozivikanwa kuti pakuenzanisa pasi pemamiriro akajairwa (750 mmHg uye 25°C), 1 L yemvura yakachena ine. at the mole
y
at the mole
ions, saka, mvura pachiyero tembiricha uye pressure (STP) ine pH ye7.
Zvekuita kana iyo pH yedziva redu ZVISINA kudzorwa

Ziva yakakwira pH dziva mhedzisiro uye zvikonzero zveyakakwira pH mudziva rako
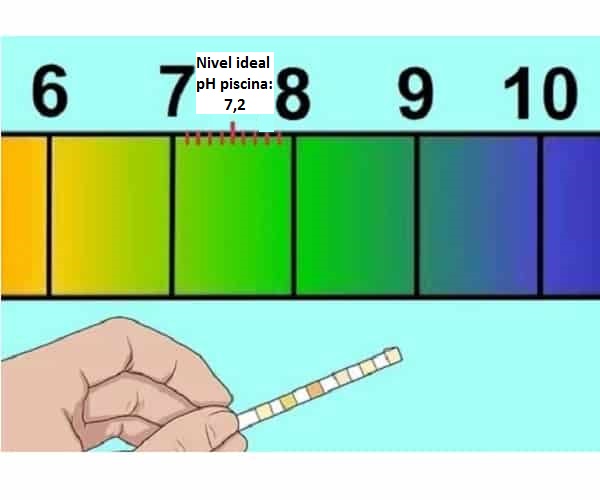
Nzira yekusimudza pH yedziva uye chii chinoitika kana yakaderera

Maitiro ekudzikisa Yepamusoro kana Alkaline Dziva pH
Nhungamiro dzemaitirwo ekugadzirisa dziva kuwedzera kune pH: kuchenesa mvura uye disinfection

Inobatsira gwara kuziva nzira yekuchenesa dziva

Nhungamiro yekuchengetedza dziva nemvura mune yakakwana mamiriro
Asidhi, kusarerekera uye alkaline pH kukosha
Kurongwa kweChiyero chepH Values
Chii chinonzi pH values

Chiyero chepH chinoenda kubva pa1 kusvika pa14, nepH 7 iri mhinduro isina kwayakarerekera.
Saka, zvinoitika kuti pH kukosha kunoratidzwa pachiyero che logarithmic pakati pehukoshi 0 (yakanyanya acidic) uye 14 (yakanyanya alkaline); Pakati tinowana kukosha 7 kwakanyorwa sekwakarerekera.
pH chiyero chepasi rose pH chiratidzo
Zvinorevei kuti chinhu chine acidic kana alkaline pH level?
Chii chinonzi acids uye mabhesi?
Acids uye mabhesi zvinhu zviripo muzvisikwa uye zvinosiyaniswa neiyo pH level, kureva, nedhigirii re acidity kana alkalinity. Maonerwo ekuti zvinhu zvine acidic here kana kuti alkaline zvinodzorwa nechiyero cheasidhi kana alkalinity inoyerwa kuburikidza neph scale uye inotangira pa0 (yakanyanya acidic kusvika pa14 (yakanyanya alkaline).Zvisinei, zvese zviri zviviri zvinhu zvinoparadza, kazhinji zvine chepfu, izvo zvisinei vane akawanda maindasitiri neanoshandiswa nevanhu.
Chii chinonzi acidic zvinhu?
- Acid pH level: pH isingasviki 7
Zvinorevei kuti pH kukosha ine acidic?
- Kuti chinhu chine acidic zvinoreva kuti chakapfuma muH+ (hydrogen ions): pH yakakura kupfuura 7
- Saka, Asidhi zvinhu zvine pH isingasviki 7. (pH yemvura yakaenzana ne7, inofungidzirwa kuti haina kwayakarerekera), iyo kemikari yacho inowanzova neayoni akawanda ehydrogen pakuwedzera mvura. Vanowanzo kuita nezvimwe zvinhu nekurasikirwa nemaproton (H+).
Ndezvipi zvinhu zvisina kwazvakarerekera?
- Neutral pH kukosha: pH yakaenzana ne7-
Zvinorevei kuti pH kukosha haina kwayakarerekera?
- pH chiyero chekuti mvura ine acidic sei/yakanyanya sei.
- Mutsara unobva pa0 kusvika pa14, uye 7 asina kwaakarerekera.
Chii chinonzi alkaline substances?
- Zvinhu zvine base kana alkaline pH: pH yakakura kupfuura 7.
Zvinorevei kana pH kukosha iri alkaline?
- Kuti chinhu chine alkaline zvinoreva kuti chakashata muH+ (kana kupfuma muOH mabhesi-, izvo zvinoita kuti H+).
- Kune izvi zvese, Mabhesi, kune rumwe rutivi, zvinhu zvine pH yakakura kupfuura 7., iyo mumhinduro dzine aqueous inowanzopa hydroxyl ions (OH-) pakati. Vanowanzova maoxidants ane simba, ndiko kuti, vanobatana nemaproton kubva kune yakatenderedza svikiro.
Kusiyana pakati pepH uye pOH kukosha

Ane hukama sei uye ndeupi musiyano uripo pakati pezviyero zveph uye poh?
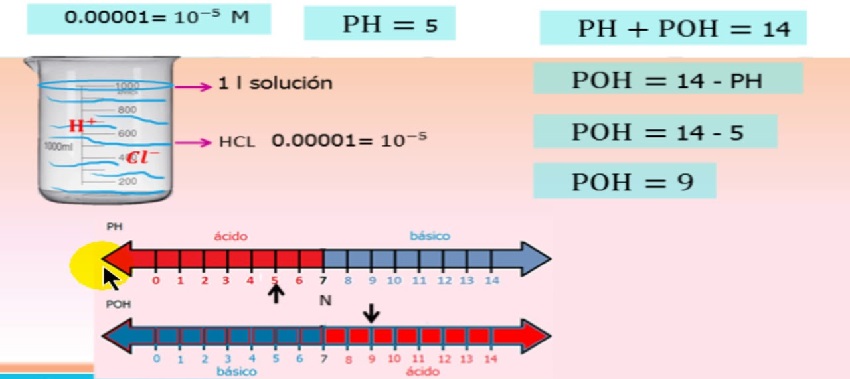
Ehe, basa reiyoni rinoenderana neiyo ion concentration uye izvi zvinotsanangurwa muequation
pH/poH ion chiitiko equation
kupi, - hydrogen ion basa
- basa coefficient yehydrogen ion
- hydrogen ion concentration
Iyo yechiitiko coefficient ibasa reiyo ion concentration uye inosvika 1 sezvo mhinduro inowedzera uye inowedzera kuderera.
Kudilute (yakanaka) mhinduro, iyo yakajairwa mamiriro eiyo solute i1,00 M, saka iyo molarity yakaenzana nebasa rayo.
Nekudaro, kune mazhinji matambudziko anofunga mhinduro dzakakodzera, isu tinogona kushandisa logarithm kune hwaro hwegumi hweiyo molar concentration, kwete chiitiko.
Misiyano pakati peiyo kukosha kwepH uye pOH
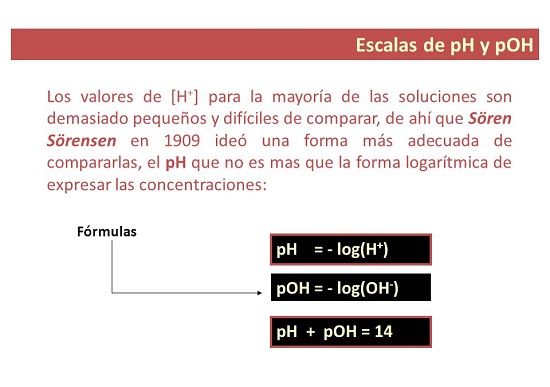
Chii chinonzi pH kukosha?
- Neimwe nzira, pH chiyero icho inoshandiswa kumisa mwero we acidity kana alkalinity yemhinduro. Izwi rekuti “p” rinomirira “kugona”, ndosaka pH ichinzi: kugona kwehydrogen.
Chii chinonzi pOH kukosha?
- Nekuda kwako. pOH chiyero chehuwandu hwehydroxyl ions mumushonga. Inoratidzwa sehwaro 10 negative logarithm yehydroxyl ion concentration uye, kusiyana nepH, inoshandiswa kuyera alkalinity level yemhinduro.

Ko pH kana pOH kukosha kunoverengwa sei?
Ndeipi formula ye ph scale values?
- Sezvainozivikanwa kare, mundima yesainzi, iyo pH ndicho chiyero de ions mukati de mhinduro. Ungatofanira kudaro kuverenga pH zvichibva pakufungisisa. calculator the pH Kushandisa equation ye pH: pH = -log[H3O+].
Ndeipi fomula yekuverenga pOH?
- Zvakare iyo pOH (kana OH kugona) chiyero cheiyo basicity kana alkalinity yemhinduro. Zvakare se inoshandisa pH = - log [H3O+] kuyera huwandu hwemaioni ehydronium [H3O+].

Key Equations kuverenga pH kana pOH kukosha
- pH=−log[H3O+]
- pOH=−log[OH-]
- [H3O+] = 10-pH
- [oh-] = 10-pOH
- pH + pOH =pKw = 14.00 pa25 °C.
Ndeupi musiyano uripo pakati pechiyero chepH kukosha uye icho chepOH?
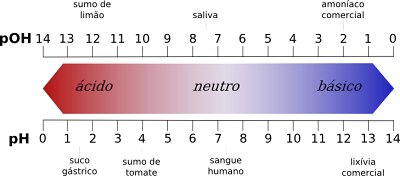
Kusaenzana pakati pezvakakosha zveiyo pH chiyero
- Kune rimwe divi, chiyero chepH chinopa maasidhi kubva pa1 kusvika pa6 nepo pOH chiyero ichipa maasidhi kubva pa8 kusvika 14.
- Sezvineiwo, chiyero chepH chinopa zvakakosha kukosha kubva pa8 kusvika 14, nepo pOH chiyero ichipa zvakakosha kukosha kubva pa1 kusvika 6.
Logarithm chiyero chehukama hwe ph uye pOH nemaitiro avo
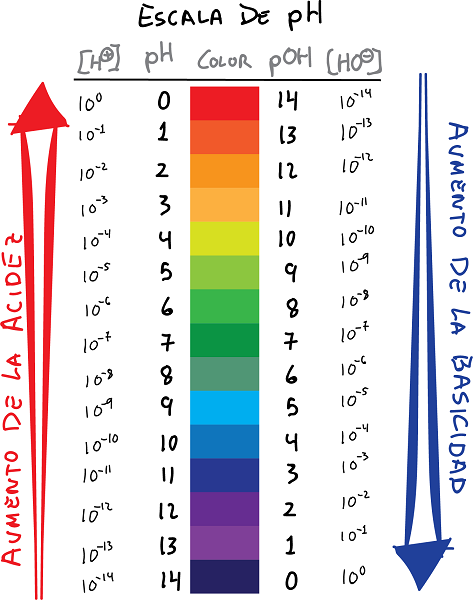
ph uye pOH chiyero chekubatana nemavara uye kukosha
- pH ndiyo logarithm yekusangana kweH ions+, nechiratidzo chakachinjwa:
- Saizvozvowo, tsanangura pOH seiyo logarithm yeOH ion concentration-, nechiratidzo chakashandurwa: Hukama hunotevera hunogona kusimbiswa pakati pe pH and the pOH.
- Chaizvoizvo, iyo pH kukosha inopa iyo yakaipa logarithm yeiyo hydrogen ion concentration, nepo pOH kukosha ichipa iyo yakaipa logarithm yeiyo hydroxide ion concentration.
Musiyano pakati pechiyero chepH uye pOH kukosha
Kusiyana pakati peiyo ph kukosha tafura uye pOH kukosha
Mushure meizvozvo, tinokupa iwe bhaisikopo raunogona kuona kuti pH inoyera kudzika kweiyo hydrogen ion, nepo pOH inoyera kudzika kwehydroxyl anions kana hydroxide ions.


