En Ọk Pool Reform, na ngalaba a n'ime ọdọ mmiri igwu mmiri pH Anyị ga-aza ajụjụ a: Kedu ihe acidic na pH bụ isi pụtara?
Index nke ọdịnaya ibe
Gịnị bụ pH na ọdọ mmiri na kedu ka ọkwa ya kwesịrị ịdị?

Kedu ihe pH dị mma pụtara maka ọdọ mmiri (7,2-7,4)
Acronym pH na-anọchi anya hydrogen nwere ike ma bụrụ ihe na-egosi acidity ma ọ bụ isi mmiri.
Ya mere, pH na-ezo aka n'ikike nke hydrogen, uru nke kwekọrọ na ntinye nke ion hydrogen na mmiri dị n'ime ọdọ mmiri gị na ya mere ọnụọgụ nke na-egosi ogo acidity ma ọ bụ isi nke mmiri. Ya mere, pH na-ahụ maka igosi ntinye nke ion H + na mmiri, na-ekpebi àgwà acidic ma ọ bụ isi.
Ọnụ ọgụgụ nke pH ụkpụrụ nke igwu mmiri mmiri

Kedu ụkpụrụ nke nha nha pH mmiri ọdọ mmiri gụnyere?
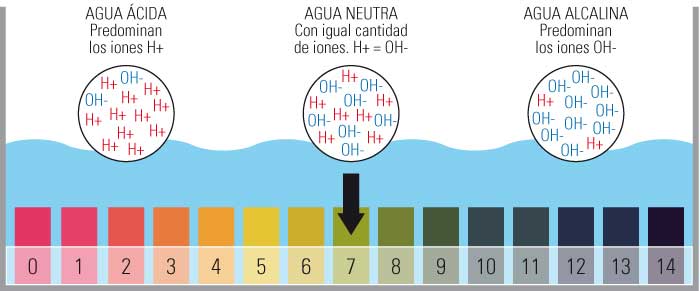
- Ọnụ ọgụgụ pH gụnyere ụkpụrụ sitere na 0 ruo 14.
- Karịsịa ịbụ 0 kachasị acidic, 14 kacha nke bụ isi yana idobe pH nọpụ iche na 7.
- A na-ekpebi nha a site na ọnụọgụ ion hydrogen efu (H+) dị na ihe ahụ.

Gịnị mere anyị chọrọ pH?
pH bụ ihe eji akọwapụta acidity ma ọ bụ ntọala nke ngwọta mmiri. Ma ihe ngwọta mmiri na-emeghachi omume dị ka acid ma ọ bụ ntọala dabere na ọdịnaya nke ion hydrogen (H+).
Otú ọ dị, ọbụna kemịkal dị ọcha na mmiri na-anọpụ iche nwere ụfọdụ ion hydrogen n'ihi ikewapụ onwe ya nke mmiri.
A maara na na nha nha n'okpuru ọnọdụ ọkọlọtọ (750 mmHg na 25 ° C), 1 L nke mmiri dị ọcha nwere. juru
y
juru
ions, ya mere, mmiri na ọnọdụ okpomọkụ na nrụgide (STP) nwere pH nke 7.
Ihe ị ga-eme mgbe pH nke ọdọ mmiri anyị anaghị achịkwa ya

Mara nnukwu pH ọdọ mmiri pụta yana ihe kpatara oke pH dị na ọdọ mmiri gị
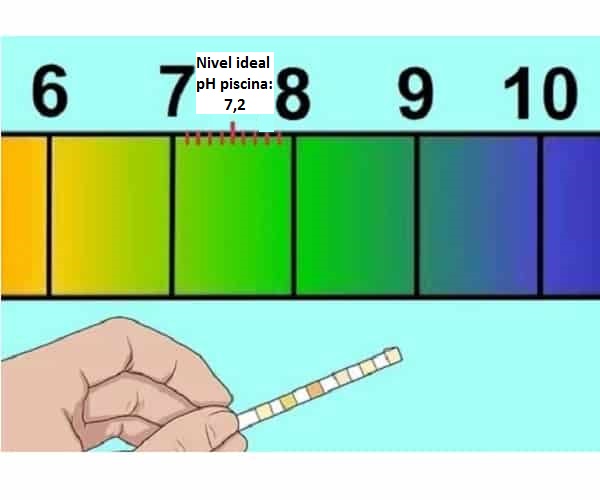
Otu esi ebuli pH nke ọdọ mmiri na ihe na-eme ma ọ bụrụ na ọ dị ala

Otu esi eweda ọdọ mmiri dị elu ma ọ bụ Alkaline pH
Ntuziaka maka otu esi eme mmezi ọdọ mmiri na mgbakwunye na pH: nhicha mmiri na disinfection

Ntuziaka bara uru ịmara ka esi ehicha ọdọ mmiri

Ntuziaka maka idobe ọdọ mmiri na mmiri na ọnọdụ zuru oke
Kedu ka pH nke ngwọta nwere ike ịbụ?

pH nke ngwọta
pH na-anọchi anya "ike hydrogen" ma ọ bụ "ike nke hydrogen." pH bụ ihe na-adịghị mma nke isi 10 logarithm nke ọrụ hydrogen ion.
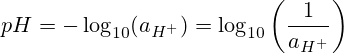
Otú ọ dị, n'ọtụtụ nsogbu kemịkalụ, anyị anaghị eji ọrụ nke ion hydrogen, kama ọnyà molar ma ọ bụ molarity.

Kedu ka ngwọta pH dị iche iche si dị
Iji malite, ị kwesịrị ịma na ọnụ ọgụgụ pH bụ logarithmic.
Ya mere, ọ pụtara na ihe dị iche site na otu pụtara ọdịiche site n'usoro nke ịdị ukwuu, ma ọ bụ ugboro iri na inversely na-egosi ịta nke hydrogen ion na ngwọta.
Ya mere, pH dị ala na-egosi ọkwa dị elu nke ion hydrogen na nke ọzọ.

Gịnị bụ acid na ntọala ogige na pH
Acid siri ike na ntọala siri ike bụ ogige nke, maka ebumnuche niile bara uru, na-ekewa kpamkpam n'ime ion ha na mmiri.
N'ihi ya, ntinye nke ion hydrogen na ngwọta ndị dị otú ahụ nwere ike ịre nhata na ntinye nke acid.
Ngụkọta pH na-adị mfe
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Ngụkọta pH site na iji mkpokọta molar dị iche iche maka acid siri ike / isi na acid na-adịghị ike.
Acid, nnọpụiche na ụkpụrụ pH alkaline
Nhazi nke Ọkwa nke Uru pH

Gịnị bụ pH ụkpụrụ

Ọnụ ọgụgụ pH na-esi na 1 ruo 14, yana pH 7 bụ ngwọta na-anọpụ iche.
Ya mere, ọ na-apụta na pH bụ uru nke egosipụtara na ọnụ ọgụgụ logarithmic n'etiti ụkpụrụ 0 (oke acidic) na 14 (oke alkaline); N'etiti anyị na-ahụ uru 7 katalọgụ dị ka nnọpụiche.
pH ọnụ ọgụgụ eluigwe na ala pH egosi

Kedu ihe ọ pụtara na ihe nwere ọkwa acidic ma ọ bụ alkaline pH?
Kedu ihe bụ acid na bases?
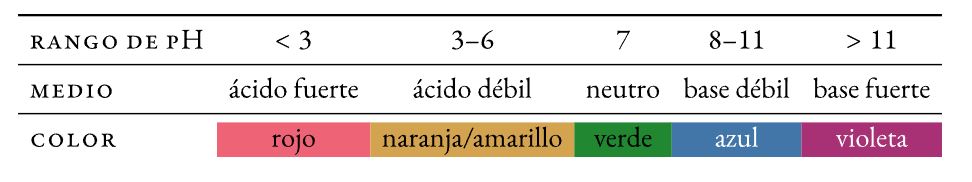
Acids na bases bụ ihe ndị dị na okike ma na-amata ya site na ọkwa pH ha, ya bụ, site na ogo acidity ma ọ bụ alkalinity. A na-achịkwa mkpebi nke ma ihe ọ bụ acidic ma ọ bụ alkaline site na ogo acidity ma ọ bụ alkalinity a tụrụ site na nha pH wee malite site na 0 (oke acidic ruo 14 (oke alkaline). ka o sina dị nwere ọtụtụ ụlọ ọrụ mmepụta ihe na mmadụ ngwa.
Kedu ka esi ahazi ihe ndị dabere na nha nke ụkpụrụ pH
Nhazi nke ihe dị na acids ma ọ bụ alkalines dịka uru pH siri dị
N'otu aka ahụ, acidity na alkalinity bụ okwu abụọ na-anabata ụzọ nke nhazi mmeghachi omume nke mmewere ọ bụla.

- N'otu aka ahụ, anyị na-esi ọnwụ ọzọ, Ọnụ ọgụgụ pH na-esi na 1 ruo 14, yana pH 7 bụ ngwọta na-anọpụ iche.
- Ọ bụrụ na pH erughị 7, ngwọta bụ acidic., ka acid na-abawanye uru pH na-ebelata maka nke ahụ a acid bụ ihe kemịkal ahụ nwere ike inye protons (H+) na chemical ọzọ.
- Kama nke ahụỌ bụrụ na pH karịrị 7, a na-akpọ ihe ngwọta nke isi (ma ọ bụ alkaline) na ọ ga-abụ ihe ndị ọzọ isi na elu ya pH; na dị ka egosiri isi bụ ihe kemịkal ahụ nwere ike ijide proton (H+) nke ọzọ chemical.
Kedu ihe bụ alkaline ma ọ bụ nke bụ isi dị ka nha pH si dị
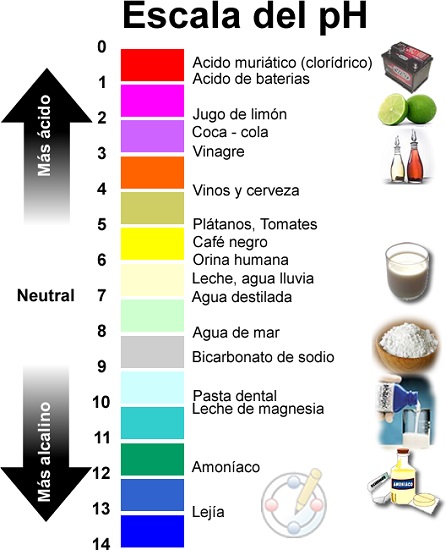
Kedu ihe bụ acidic?
- Acid pH: pH erughị 7
Kedu ihe ọ pụtara na pH uru bụ acidic?
- Na ihe bụ acidic pụtara na ọ bara ụba na H+ (hydrogen ions): pH karịrị 7
- N'ihi ya, Acids bụ ihe nwere pH na-erughị 7. (pH mmiri hà nhata 7, a na-ewere na-anọpụ iche), onye kemistri ya na-enwekarị nnukwu ion hydrogen mgbe ọ na-agbakwunye mmiri. Ha na-emeghachi omume na ihe ndị ọzọ site na ịhapụ protons (H+).
Kedu ihe bụ ihe na-anọpụ iche?
- Uru pH na-anọpụ iche: pH hà 7-
Kedu ihe ọ pụtara na uru pH na-anọpụ iche?
- pH bụ ihe nleba anya ka mmiri si dị / isi.
- Usoro ahụ sitere na 0 ruo 14, ebe 7 na-anọpụ iche.
Kedu ihe bụ alkaline bekee?
- Ihe nwere ala ma ọ bụ alkaline pH: pH karịrị 7.
Kedu ihe ọ pụtara mgbe uru pH bụ alkaline?
- Na ihe bụ alkaline pụtara na ọ dara ogbenye na H+ (ma ọ bụ ọgaranya na ntọala OH-, nke na-ewepụ ihe H+).
- Maka ihe a niile, Bases, n'aka nke ọzọ, bụ ihe nwere pH karịa 7., nke na ngwọta mmiri na-enyekarị ion hydroxyl (OH-) n'etiti. Ha na-abụkarị ndị dị ike oxidants, ya bụ, ha na-emeghachi omume na protons sitere na gburugburu gburugburu.
Kedu ihe bụ acidity na alkalinity?
Kedu ihe bụ acidity na alkalinity na nri
Mgbe ahụ, na vidiyo a, a ga-agwa gị maka ọnụọgụ nri na-adịghị agwụ agwụ nke anyị na-eri kwa ụbọchị mana,
- Ọ dịtụla mgbe ị nọ na-eche ihe mere ụfọdụ ekpomeekpo na-adọta uche anyị karịa ndị ọzọ?
- Anụ ụtọ dị ka nnu, achịcha, ihe ọṅụṅụ dị nro, ihe ọṅụṅụ, ọbụna soseji.
- Maka gịnị bụ ihe a?
- Anyị ga-akọwara gị ihe niile a na ihe ndị ọzọ ugbu a na ndekọ.
Theories nke acidic na isi pH

Atụmatụ acid-base nke pH
Kedu ihe bụ Arrhenius pH Theory?

Ndị Swedish tụrụ aro ya Svante Arrhenius na 1884, bụ nkọwa nke mbụ ọgbara ọhụrụ nke acids na bases na okwu molecular.
Arrhenius acid ph theory
Ihe na-ekewa n'ime mmiri iji mepụta cations hydrogen (H+).
Arrhenius basic pH theory
Ihe na-ekewa n'ime mmiri iji mepụta anions hydroxide (OH-).
ARRHENIUS THEORY Gịnị bụ acid? Gịnị bụ ntọala?
Arrhenius acid na isi pH theory video
Brønsted-Lowry ph theory
Kedu ihe bụ theory Brønsted-Lowry nke pH?

Ndị Danish tụrụ aro na 1923 n'adabereghị Johannes Nicolaus Bronsted na Bekee Martin lowry, dabere na echiche nke conjugate acid-base ụzọ abụọ.
Mgbe acid, HA, na-emeghachi omume na isi, B, acid ahụ na-etolite ntọala conjugate ya, A.-, na isi mejupụtara ya conjugate acid, HB+, site n'ịgbanwe proton (cation H+):
HA+B⇌A-+HB+
Brønsted-Lowry acid ph theory
Ihe pH acid: ike inye protons (H+) na ndabere:
HA+H2O⇌A-+H3O+
Usoro pH bụ isi Brønsted-Lowry
Ihe nwere pH bụ isi: ike ịnakwere protons (H+) nke acid:
B+H2O⇌HB++OH-
Nke a tiori a na-atụle a izugbe nke tiori nke Arrhenius.
BRÖNSTED-LOWRY THEORY Gịnị bụ acid? Gịnị bụ ntọala?
vidiyo pH BRÖNSTED-LOWRY
Nkọwa ọrụ nke nha pH enwere ike

Kedu ihe bụ ACIDITY na ALKALINITY?
Kedu ihe acidic na pH bụ isi pụtara?

acid pH
- Na mbụ, anyị nwere ike ịchọta ihe ngwọta na pH acidic: ihe na-eme ka akwụkwọ na-acha anụnụ anụnụ na-acha uhie uhie, na-emeghachi na ụfọdụ ọla, na-emepụta nnu ma na-ahapụ hydrogen (mmeghachi omume exothermic).
- Na mgbakwunye, ihe nwere pH acidic na-agbazinye uru n'etiti 0 na 7.
isi pH uru

- Nke abụọ, e nwere ndị Base pH: Ihe na-atụgharị na-acha uhie uhie akwụkwọ litmus acha anụnụ anụnụ wee tụgharịa pink mgbe ejiri phenolphthalein meghachi omume.
- N'aka nke ọzọ, gosi na ha nwere uru pH n'etiti 7 na 14.
anọghị pH

- N'ikpeazụ, ihe nwere nha pH na-anọpụ iche bụ nke na-adịghị emeghachi omume na ihe ngosi acid-base.
- Ọzọkwa, pH nke ihe ndị a hà nhata 7.
Ihe ndị nwere pH acid siri ike


Ntụle nke ngwọta acid na pH
Kedu ka ụkpụrụ acidic dị na pH
- Acids na-ahapụ ion hydrogen, yabụ mmiri mmiri ha nwere ion hydrogen karịa mmiri na-anọpụ iche ma na-ewere ya dị ka acidic n'okpuru pH 7.
Kedu ngwaahịa pH acid siri ike na-ahụkarị
Enwere naanị acid asaa siri ike:
- - hydrochloric acid HCl
- nitric acid HNO3
- - sulfuric acid H2SO4
- - hydrobromic acid HBr
- - HI hydroiodic acid
- - perchloric acid HClO4
- - chloric acid HClO3

Usoro pH acid siri ike
usoro pH acid siri ike
Usoro pH acid siri ike: [HNO3] = [H3O+], na pH = -log[H3O+].
Gbakọọ acid siri ike ph online
Gbakọọ pH nke ngwọta acid siri ike.
Ihe ndị nwere pH isi siri ike

Ntụle nke isi ihe ngwọta na pH

Kedu ka ụkpụrụ acidic dị na pH
Ihe eji mara ya na pH isi
- Bases na-anabata ion hydrogen (jikọta na ụfọdụ ion hydrogen guzobere site na nkesa mmiri), yabụ mmiri mmiri ha nwere ion hydrogen pere mpe karịa mmiri na-anọpụ iche ma na-ewere ya dị ka isi n'elu pH 7.

Usoro iji gbakọọ pH ntọala siri ike
usoro pH acid siri ike
Usoro pH acid siri ike: [HNO3] = [H3O+], na pH = -log[H3O+].
Kedu ngwaahịa pH acid siri ike na-ahụkarị
Enweghịkwa ọtụtụ ntọala siri ike, ụfọdụ n'ime ha anaghịkwa agbaze na mmiri. Ndị soluble bụ

- - sodium hydroxide NaOH
- - potassium hydroxide KOH
- - lithium hydroxide liOH
- - rubidium hydroxide RbOH
- - Csium hydroxide CsOH
Mgbakọ pH siri ike
Mgbakọ nke pH isi siri ike
Ihe na usoro nwere acidic na-adịghị ike ma ọ bụ pH bụ isi

Kedu ka ụkpụrụ pH si bụrụ acid / isi adịghị ike
Isi njirimara nke acids na bases na-adịghị ike bụ na ha na-ekewa n'ime mmiri. Ewubere nha nha n'etiti usoro na-aga n'ihu na ntụgharị, na-erute ọnọdụ kwụ ọtọ nke ogo nkewa na-adabere na ike nke acid ma ọ bụ isi.

Acids/bases adịghị ike na-ekewa n'ime mmiri. Ịchọta pH nke acid na-adịghị ike bụ ntakịrị mgbagwoju anya.

Ụdị pH acid adịghị ike
usoro pH acid na-adịghị ike
Nha nhata pH ka dị otu: , ma ị ga-eji acid dissociation mgbe niile (Ka) ịchọta [H+].
Usoro maka Ka bụ:
ebe: - ịta nke H + ion
- ịta nke conjugated isi ion
- ịta nke mkpụrụ ndụ acid na-enweghị ihe ọ bụla
maka mmeghachi omume
Gbakọọ pH nke ngwọta acid na-adịghị ike.
Gbakọọ pH nke ngwọta acid na-adịghị ike.
Usoro pH isi adịghị ike
Formula iji nweta pH nke isi adịghị ike
Kedu ka esi agbakọọ pH nke ntọala adịghị ike?
Mgbe ị nwetasịrị pOH site na usoro pOH dị n'elu, a pH ị nwere ike gbakọọ iji usoro pH =pKw - pOH ebe pK w = 14.00.
Ọdịiche dị n'etiti ihe bụ uru pH na pOH
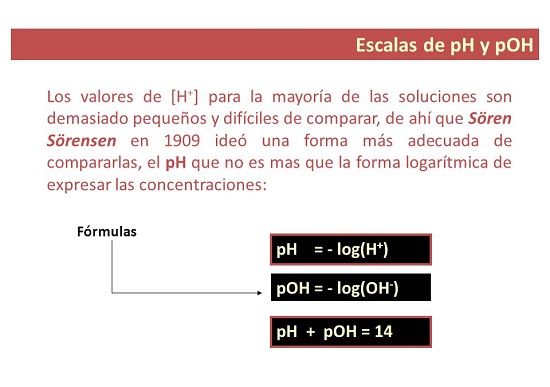
Kedu uru pH nkịtị?
- N'otu aka ahụ, pH bụ nha nke ahụ A na-eji iji guzobe ọkwa nke acidity ma ọ bụ alkalinity nke ngwọta. "p" na-anọchi anya "nwere ike", nke mere e ji kpọọ pH: ikike nke hydrogen.
Gịnị bụ uru pOH?
- Maka akụkụ nke gị. pOH bụ ihe nleba anya nke ion hydroxyl na ngwọta. A na-egosipụta ya dị ka isi 10 logarithm na-adịghị mma nke mkpokọta hydroxyl ion na, n'adịghị ka pH, a na-eji tụọ ọkwa alkalinity nke ngwọta.
Gbakọọ pH ntọala adịghị ike
Mgbakọ nke pH isi adịghị ike
Ike nke Acids na ntọala

Ọdịiche dị n'etiti acidic siri ike na adịghị ike na pH bụ isi
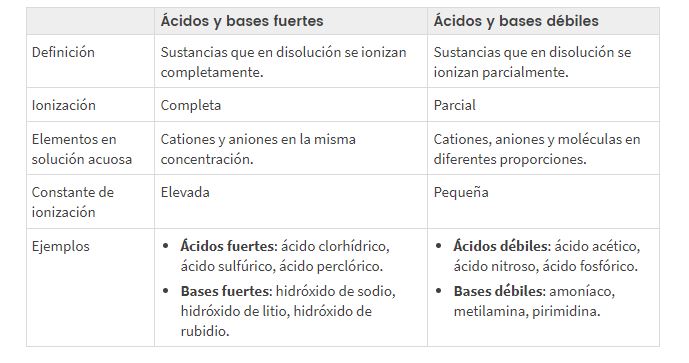
Kedu ihe nhazi nke acidic siri ike na nke na-adịghị ike na pH bụ isi dabere na?
Dabere n'otú ionized ma ọ bụ kewaa acid ma ọ bụ isi, anyị na-ama ọdịiche dị n'etiti acids/bases siri ike na adịghị ike, okwu ndị na-akọwa mfe na ịkwọ ụgbọala la ọkụ eletrik (n'ihi na nnukwu ma ọ bụ obere ọnụnọ nke ion na ngwọta).
Acids siri ike na adịghị ike na nhazi ọkwa, ogo nkewa na ihe atụ pH
Nhazi pH adịghị ike yana acid siri ike na bastion
Ọkwa nke ionization nke acidic na isi pH
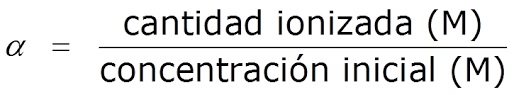
Gịnị bụ ogo nke ionization ma ọ bụ dissociation nke acidic na isi pH
A na-akpọkwa ya ogo nke dissociation, α, ka akọwara dị ka oke n'etiti ọnụọgụ acid/base ionized na ọnụọgụ nke mbụ acid/base:
ááα = ego nke ionized acid/base/ego nke mbụ acid/base
A na-egosipụtakarị ya dị ka pasenti (%).
Kedu ihe ogo ionization ma ọ bụ nkewa nke acidic na pH bụ isi pụtara?
acid siri ike na ntọala
Ionized zuru oke (α≈1). Ha na-eduzi ọkụ eletrik nke ọma.
- Acid: HClO4, HI (aq), HBr (aq), HCl (aq), H2SO4 (1st ionization) na HNO3.
- Ntọala: Hydroxides nke alkali na alkaline ụwa ọla.
Acids na bases adịghị ike
Akụkụ ionized: α <1. Ha na-eduzi ọkụ eletrik adịghị mma.
- Acid: HF (aq), H2S (aq), H2CO3, H2SO3, H3PO4, H.N.O.2 na organic acids, dị ka CH3KWU
- Ihe ndabere: NH3 (ma ọ bụ NH4OH) na ihe ndị dị na nitrogenous organic bases, dị ka amines.
Nkewa pH acids na bases mgbe niile
Gịnị bụ dissociation mgbe nile nke isi na acidic pH?
Ọ bụ ihe atụ nke ike nke a acid/base na ngwọta:
| Acid | Akara | |
|---|---|---|
| Nhazi | HA+H2O⇌A-+H3O+ | B+H2O⇌HB++OH- |
| NA-AKWỤKWỌ | Ka=[A-][H3O+][HA] | Kb=[HB+] [OH-] [B] |
| COLOGARHYTHM | pKa=-log Ka | pKb=-logKb |
Ike ikwu nke acidic na pH bụ isi
Acid na isi pH mgbe niile
Ion itule nke mmiri

Isi: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Kedu ihe bụ amphoteric
amphoteric kedu ihe ha bụ
Na kemistri, ihe amphoteric bụ nke nwere ike meghachi omume dị ka acid ma ọ bụ ntọala.;
kedu ebe okwu si bia amphoteric
Okwu a sitere na prefix Greek amphi- (αμφu-), nke putara 'ha abuo'. Ọtụtụ ọla (dị ka zinc, tin, lead, aluminum, na beryllium) na ọtụtụ metalloids nwere. oxides ma ọ bụ hydroxides amphoteric.

Mmiri bụ ihe amphiprotic
Kedu ihe ọ pụtara na mmiri bụ ihe amphiprotic
El mmiri bụ ihe amphiprotic (nwere ike inye onyinye ma ọ bụ nabata proton H+), nke na-enye ohere ka ọ rụọ ọrụ dị ka acid ma ọ bụ isi (amphotericism).
Usoro nguzozi mmiri ionic

El ionic itule nke mmiri na-ezo aka mmeghachi omume kemịkalụ nke ụmụ irighiri mmiri abụọ na-emeghachi omume iji mepụta ion oxonium (H3O+) na ion hydroxide (ỌH-):
Nha nhatanha mgbe niile, a na-akpọ ionic ngwaahịa mmiri, ma gosi ya site na KW, ngwaahịa a nwere ike were were were:
Kw=[H3O+][OH-]
Na 25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH na ngwaahịa ionic nke mmiri (Kw). Acid-BASE
Acid-base pH egosi

Un egosi pH bụ ngwakọta kemịkalụ halochromic (na-agbanwe agba ya -ehulata- tupu mgbanwe na pH) nke agbakwunyere na obere ihe ngwọta iji chọpụta pH ya (acidity ma ọ bụ isi). A na-akpọ mgbanwe agba tụgharịa.
Litmus
Ngwakọta mmiri soluble nke dị iche iche esiji lichens. Na-amịkọrọ n'akwụkwọ nzacha, ọ bụ otu n'ime ihe ngosi pH kacha ochie ejiri (~ 1300).

Methyl oroma
Agba azo eweputa nke na-esi na-acha uhie uhie gaa na oroma-edo edo ọkara acid:

Phenolphthalein
Ihe ngosi pH na-enweghị agba na ọkara acid nke na-atụgharị pink isi ọkara:

eluigwe na ala egosi
Ngwakọta egosi (thymol blue, methyl red, bromothymol blue, na phenolphthalein) nke na-egosipụta mgbanwe dị nro na agba n'ọtụtụ dịgasị iche iche nke ụkpụrụ pH.
Acid-base neutralization titration

Acid-base titration/titration bụ usoro nyocha kemịkalụ ọnụọgụ
Kedu usoro nyocha kemịkalụ acid na basci pH titration
una acid-base titration/titration bụ usoro nyocha kemịkalụ ọnụọgụ maka ịchọpụta mkpokọta acid ma ọ bụ isi amapụtara (nyocha), na-ewepụ ya kpọmkwem na ngwọta ọkọlọtọ nke isi ma ọ bụ acid nke itinye uche mara (obi ike).

Titration/titration curve nke 25 ml nke 0.1 M acetic acid nwere 0.1 M sodium hydroxide.
Neutralization: mmeghachi omume n'etiti ngwakọta nke acid na isi

Kedu ihe ga - eme ma ọ bụrụ na ị gwakọta acid na isi?
A na-akpọ mmeghachi omume n'etiti acid na ntọala neutralization.
- Mmeghachi omume nnọpụiche na-abụkarị exothermic. que pụtara que Ha na-enye ike n'ụdị okpomọkụ.
- Se ọ na-akpọkarị ha neutralization n'ihi na mgbe ọ na-emeghachi omume a acid na a isi,
- Ya mere, mmeghachi omume n'etiti acid na bases ka a na-akpọ neutralization. na ọtụtụ ma ọ bụ obere na-ewepụ acidic ma ọ bụ ihe ndị bụ isi nke ogige abụọ ahụ, ya bụ, ha na-ewepụ ihe onwunwe nke onye ọzọ. na-emepụta mmiri na nnu kama.
Ngwakọta nke acid na isi na-ewepụ onwe ya, pH ekwesịghị ịbụ onye na-anọpụ iche.
- Ihe kpatara na ngwakọta nke acid na base neutralizes onwe ya pH ekwesịghị ịbụ nnọpụiche n'ihi na ọ bụ ọnụọgụ acid na / ma ọ bụ isi ka a na-ekpebi pH n'ikpeazụ.
- Kama nke ahụ, Ọ bụrụ na ego nke H+ na OH- bụ otu ihe ahụ, ihe ngwọta na-aghọ onye na-anọpụ iche n'ihi na ha na-emeghachi omume na ibe ha na-emepụta mmiri (H+ + ỌH- . H20).
Dị ka àgwà nke acid na isi mmeghachi omume, a na-ekewa ikpe anọ:
- Na mbụ acid siri ike + ntọala siri ike
- acid na-adịghị ike + isi ike
- acid siri ike + isi adịghị ike
- N'ikpeazụ, acid na-adịghị ike + isi adịghị ike
Gịnị bụ mmeghachi omume neutralization pH na acidic?
Na mmeghachi omume nke neutralization, acid na isi na-emeghachi omume n'otu ụzọ ahụ enweghị ike ịgbagha iji mepụta nnu na mmiri:
Acid + BASE ⟶ Nnụ + Mmiri
Dabere na ma titrant bụ acid siri ike ma ọ bụ ntọala, pH na nha nha ga-abụ:
| ANALYTE/VALUANT | ike/ike | Acid/Ike siri ike | Isi adịghị ike/Acid siri ike |
|---|---|---|---|
| pH (EKWUKWU) | 7 | > 7 | <7 |
| Ngosipụta (tụgharịrị n'etiti) | Nnọpụiche | Nhazi | Acid |
Otu esi agbakọ pH nke Ngwọta

Kedu ihe bụ usoro maka pH?
Na sayensị, pH bụ nha nke ion na ngwọta. Ị nwere ike ịgbakọ pH dabere na ntinye uche.
Formula maka ịgbakọ pH
Gbakọọ pH site na iji nha nhata pH: pH = -log[H3O+].
Ihe mgbako pH maka ọdọ mmiri igwu mmiri
Vidiyo gbakọọ pH nke ngwọta
Na 1909, onye Danish biochemist Soren Sorensen tụrụ aro okwu pH iji gosi "nwere ike nke hydrogen ion". Ọ kọwara pH dị ka logarithm nke [H+] gbanwere na akara. Na-akọwapụta dị ka ọrụ nke [H3O+].
Ihe mgbako pH ngwọta

pH nke ihe mgbako ngwọta
Gbakọọ pH nke ngwọta
N'okpuru bụ mgbako abụọ ị nwere ike iji lelee azịza nsogbu kemịkalụ.
- Nke mbụ gbakọọ pH nke ngwọta nke acid siri ike o ntọala siri ike.
- Na, nke abụọ gbakọọ pH nke ngwọta nke acid na-adịghị ike o isi adịghị ike.
Gbakọọ pH nke ngwọta acid/base siri ike
Ihe mgbako maka pH nke ngwọta acid/base siri ike
[planetcalc cid = »8830″ asụsụ =»es» koodu =»» labelụ =»PLANETCALC, The pH nke a siri ike acid / isi ngwọta» agba =»#263238,#435863,#090c0d,#fa7014,#fb9b5a,# c25004″ v=»4165″]
Gbakọọ pH nke ngwọta acid/base adịghị ike
Ihe mgbako maka pH nke ngwọta acid/base adịghị ike
[planetcalc cid = »8834″ asụsụ =»es» koodu =»» labelụ =»PLANETCALC, The pH nke adịghị ike acid/base ngwọta» agba =»#263238,#435863,#090c0d,#fa7014,#fb9b5a,# c25004″ v=»4165″]