
Index nke ọdịnaya ibe
En Ọk Pool Reform, na ngalaba a n'ime ọdọ mmiri igwu mmiri pH anyị ga-emeso ndị ọdịiche dị n'etiti ph na poh na ụkpụrụ mmiri ọdọ mmiri.
Gịnị bụ pH na ọdọ mmiri na kedu ka ọkwa ya kwesịrị ịdị?

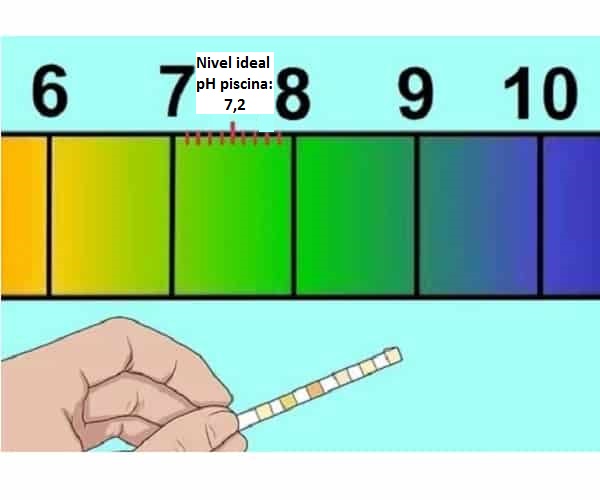
Kedu ihe pH dị mma pụtara maka ọdọ mmiri (7,2-7,4)
Acronym pH na-anọchi anya hydrogen nwere ike ma bụrụ ihe na-egosi acidity ma ọ bụ isi mmiri.
Ya mere, pH na-ezo aka n'ikike nke hydrogen, uru nke kwekọrọ na ntinye nke ion hydrogen na mmiri dị n'ime ọdọ mmiri gị na ya mere ọnụọgụ nke na-egosi ogo acidity ma ọ bụ isi nke mmiri. Ya mere, pH na-ahụ maka igosi ntinye nke ion H + na mmiri, na-ekpebi àgwà acidic ma ọ bụ isi.
Ọnụ ọgụgụ nke pH ụkpụrụ nke igwu mmiri mmiri
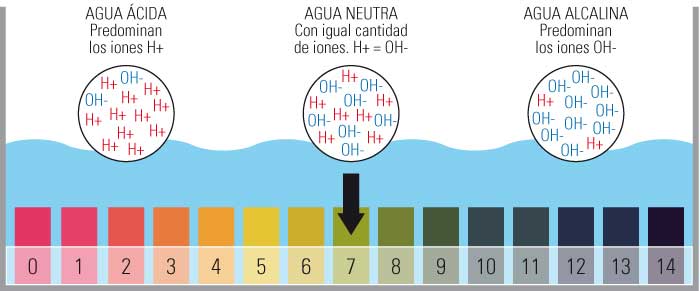

Kedu ụkpụrụ nke nha nha pH mmiri ọdọ mmiri gụnyere?
- Ọnụ ọgụgụ pH gụnyere ụkpụrụ sitere na 0 ruo 14.
- Karịsịa ịbụ 0 kachasị acidic, 14 kacha nke bụ isi yana idobe pH nọpụ iche na 7.
- A na-ekpebi nha a site na ọnụọgụ ion hydrogen efu (H+) dị na ihe ahụ.

Gịnị mere anyị chọrọ pH?
pH bụ ihe eji akọwapụta acidity ma ọ bụ ntọala nke ngwọta mmiri. Ma ihe ngwọta mmiri na-emeghachi omume dị ka acid ma ọ bụ ntọala dabere na ọdịnaya nke ion hydrogen (H+).
Otú ọ dị, ọbụna kemịkal dị ọcha na mmiri na-anọpụ iche nwere ụfọdụ ion hydrogen n'ihi ikewapụ onwe ya nke mmiri.
A maara na na nha nha n'okpuru ọnọdụ ọkọlọtọ (750 mmHg na 25 ° C), 1 L nke mmiri dị ọcha nwere. juru
y
juru
ions, ya mere, mmiri na ọnọdụ okpomọkụ na nrụgide (STP) nwere pH nke 7.
Ihe ị ga-eme mgbe pH nke ọdọ mmiri anyị anaghị achịkwa ya

Mara nnukwu pH ọdọ mmiri pụta yana ihe kpatara oke pH dị na ọdọ mmiri gị
Otu esi ebuli pH nke ọdọ mmiri na ihe na-eme ma ọ bụrụ na ọ dị ala

Otu esi eweda ọdọ mmiri dị elu ma ọ bụ Alkaline pH
Ntuziaka maka otu esi eme mmezi ọdọ mmiri na mgbakwunye na pH: nhicha mmiri na disinfection

Ntuziaka bara uru ịmara ka esi ehicha ọdọ mmiri

Ntuziaka maka idobe ọdọ mmiri na mmiri na ọnọdụ zuru oke
Acid, nnọpụiche na ụkpụrụ pH alkaline
Nhazi nke Ọkwa nke Uru pH
Gịnị bụ pH ụkpụrụ

Ọnụ ọgụgụ pH na-esi na 1 ruo 14, yana pH 7 bụ ngwọta na-anọpụ iche.
Ya mere, ọ na-apụta na pH bụ uru nke egosipụtara na ọnụ ọgụgụ logarithmic n'etiti ụkpụrụ 0 (oke acidic) na 14 (oke alkaline); N'etiti anyị na-ahụ uru 7 katalọgụ dị ka nnọpụiche.
pH ọnụ ọgụgụ eluigwe na ala pH egosi
Kedu ihe ọ pụtara na ihe nwere ọkwa acidic ma ọ bụ alkaline pH?
Kedu ihe bụ acid na bases?
Acids na bases bụ ihe ndị dị na okike ma na-amata ya site na ọkwa pH ha, ya bụ, site na ogo acidity ma ọ bụ alkalinity. A na-achịkwa mkpebi nke ma ihe ọ bụ acidic ma ọ bụ alkaline site na ogo acidity ma ọ bụ alkalinity a tụrụ site na nha pH wee malite site na 0 (oke acidic ruo 14 (oke alkaline). ka o sina dị nwere ọtụtụ ụlọ ọrụ mmepụta ihe na mmadụ ngwa.
Kedu ihe bụ acidic?
- Acid pH: pH erughị 7
Kedu ihe ọ pụtara na pH uru bụ acidic?
- Na ihe bụ acidic pụtara na ọ bara ụba na H+ (hydrogen ions): pH karịrị 7
- N'ihi ya, Acids bụ ihe nwere pH na-erughị 7. (pH mmiri hà nhata 7, a na-ewere na-anọpụ iche), onye kemistri ya na-enwekarị nnukwu ion hydrogen mgbe ọ na-agbakwunye mmiri. Ha na-emeghachi omume na ihe ndị ọzọ site na ịhapụ protons (H+).
Kedu ihe bụ ihe na-anọpụ iche?
- Uru pH na-anọpụ iche: pH hà 7-
Kedu ihe ọ pụtara na uru pH na-anọpụ iche?
- pH bụ ihe nleba anya ka mmiri si dị / isi.
- Usoro ahụ sitere na 0 ruo 14, ebe 7 na-anọpụ iche.
Kedu ihe bụ alkaline bekee?
- Ihe nwere ala ma ọ bụ alkaline pH: pH karịrị 7.
Kedu ihe ọ pụtara mgbe uru pH bụ alkaline?
- Na ihe bụ alkaline pụtara na ọ dara ogbenye na H+ (ma ọ bụ ọgaranya na ntọala OH-, nke na-ewepụ ihe H+).
- Maka ihe a niile, Bases, n'aka nke ọzọ, bụ ihe nwere pH karịa 7., nke na ngwọta mmiri na-enyekarị ion hydroxyl (OH-) n'etiti. Ha na-abụkarị ndị dị ike oxidants, ya bụ, ha na-emeghachi omume na protons sitere na gburugburu gburugburu.
Ọdịiche dị n'etiti ụkpụrụ pH na pOH

Kedu ka njikọ ha si dị na kedu ihe dị iche n'etiti nha ph na poh?
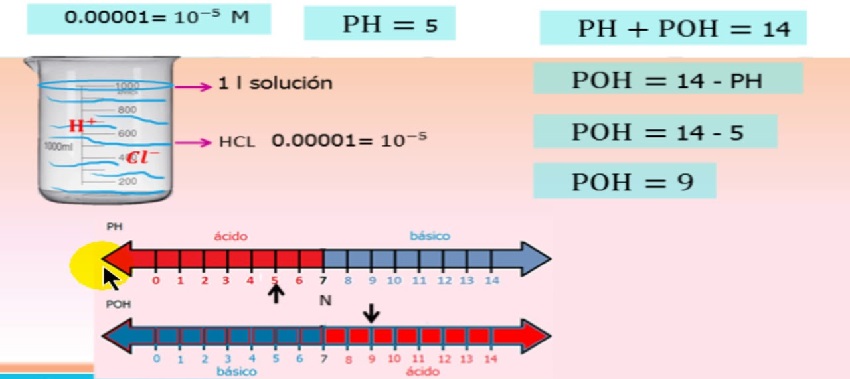
N'ezie, ọrụ nke ion na-adabere na ntinye ion na nke a na-akọwa na nha nha
pH/poH ion ọrụ nhata
Ebee, - ọrụ hydrogen ion
- ọnụọgụ ọrụ nke ion hydrogen
- hydrogen ion ịta
Ọnụ ọgụgụ ọrụ bụ ọrụ nke ntinye ion ma na-abịaru nso 1 ka ihe ngwọta na-aghọwanye ihe na-esiwanye ike.
Maka ngwọta dilute (ezigbo), ọnọdụ ọkọlọtọ nke solute bụ 1,00 M, ya mere molarity ya na-arụ ọrụ ya.
Ya mere, maka ọtụtụ nsogbu ndị na-eche ihe ngwọta dị mma, anyị nwere ike iji logarithm na isi 10 nke mkpokọta molar, ọ bụghị ọrụ ahụ.
Ọdịiche dị n'etiti ihe bụ uru pH na pOH
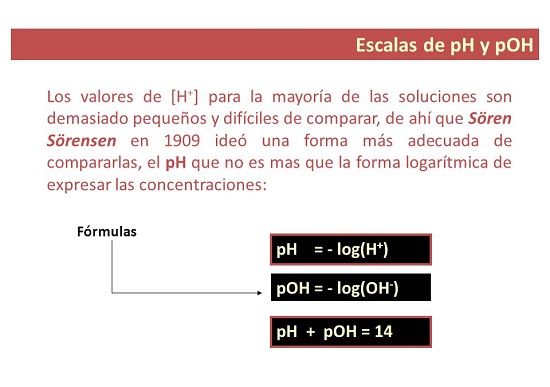
Kedu uru pH nkịtị?
- N'otu aka ahụ, pH bụ nha nke ahụ A na-eji iji guzobe ọkwa nke acidity ma ọ bụ alkalinity nke ngwọta. "p" na-anọchi anya "nwere ike", nke mere e ji kpọọ pH: ikike nke hydrogen.
Gịnị bụ uru pOH?
- Maka akụkụ nke gị. pOH bụ ihe nleba anya nke ion hydroxyl na ngwọta. A na-egosipụta ya dị ka isi 10 logarithm na-adịghị mma nke mkpokọta hydroxyl ion na, n'adịghị ka pH, a na-eji tụọ ọkwa alkalinity nke ngwọta.

Kedu ka esi agbakọ uru pH ma ọ bụ pOH?
Gịnị bụ usoro maka ụkpụrụ ph scale?
- Dị ka ọ na-ama mara, na ndị ọkà mmụta sayensị ubi, na pH bụ ihe atụ de ions n'ime de ihe ngwọta. Ị nwere ike gbakọọ pH dabere na ịta ahụhụ. Gbakọọ ihe pH Iji nhata nke pH: pH = -log [H3O+].
Kedu usoro iji gbakọọ pOH?
- Ọzọkwa, na pOH (ma ọ bụ ikike OH) bụ ihe nleba anya nke ntọala ma ọ bụ alkalinity nke ngwọta. Ọzọkwa se na-eji pH = - log [H3O+] iji tụọ mkpokọta ion hydronium [H3O+].

Ndekọ igodo iji gbakọọ uru pH ma ọ bụ pOH
- pH=-log[H3O+]
- pOH=-log [OH-]
- [H3O+] = 10-pH
- [oh-] = 10-pOH
- pH + pOH =pKw = 14.00 na 25 Celsius.
Kedu ihe dị iche n'etiti ọnụ ọgụgụ pH na nke pOH?
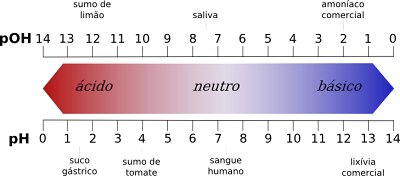
Enweghi oke n'etiti ụkpụrụ nke pH
- N'otu aka ahụ, ọnụ ọgụgụ pH na-enye ụkpụrụ acid site na 1 ruo 6 ebe ọnụ ọgụgụ pOH na-enye ụkpụrụ acid site na 8 ruo 14.
- N'aka nke ọzọ, ọnụ ọgụgụ pH na-enye ụkpụrụ ndị bụ isi site na 8 ruo 14, ebe ọnụ ọgụgụ pOH na-enye ụkpụrụ ndị bụ isi site na 1 ruo 6.
Njikọ ọnụ ọgụgụ Logarithm nke ph na pOH yana ụkpụrụ ha
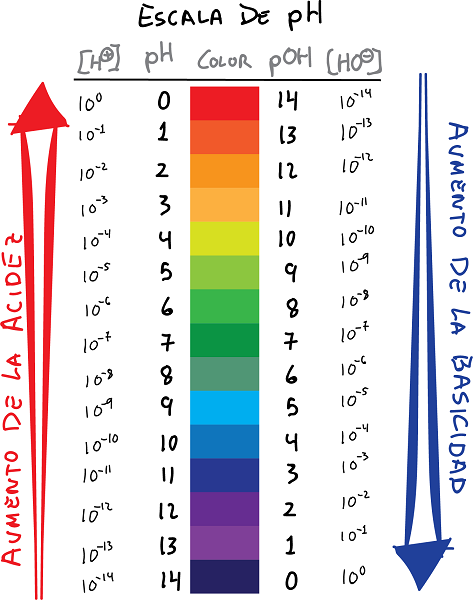
njikọ ph na pOH nwere agba na ụkpụrụ
- pH bụ logarithm nke ịta nke H ion+, na akara gbanwere:
- N'otu aka ahụ, kọwaa pOH dị ka logarithm nke ntinye uche OH ion-, na akara gbanwere: Enwere ike ịmekọrịta mmekọrịta n'etiti ndị pH na pOH.
- N'ụzọ bụ isi, ụkpụrụ pH na-enye logarithm na-adịghị mma nke mkpokọta hydrogen ion, ebe uru pOH na-enye logarithm na-adịghị mma nke mkpokọta ion hydroxide.
Ọdịiche dị n'etiti ọnụ ahịa pH na pOH
Ndịiche dị n'etiti ọnụ ahịa ph na uru pOH
Mgbe nke ahụ gasị, anyị na-enye gị ihe nkiri ebe ị ga-ahụ na pH na-atụle mkpokọta ion hydrogen, ebe pOH na-atụle mkpokọta hydroxyl anions ma ọ bụ ion hydroxide.


