En Ok Pool Reform, in this section within the Swimming pool pH level We will address the following question: What does acidic and basic pH mean?
Table of contents of the page
What is pH in a pool and what should its levels be?

What does ideal pH for swimming pools mean (7,2-7,4)?
The acronym pH means hydrogen potential and is a measure that indicates the acidity or basicity of water.
Then, pH refers to the hydrogen potential, a value that corresponds to the concentration of hydrogen ions in the water of your pool and consequently is the coefficient that indicates the degree of acidity or basicity of the water. Therefore, pH is responsible for indicating the concentration of H+ ions in water, determining its acidic or basic character.
Pool water pH value scale
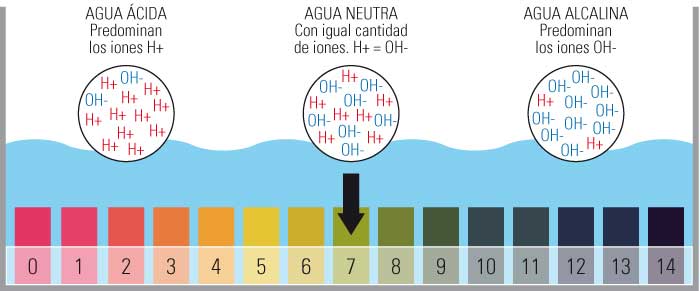

What values does the pool water pH measurement scale include?
- The pH measurement scale includes values from 0 to 14.
- Particularly, 0 being the most acidic, 14 being the most basic and placing the Neutral pH at 7.
- This measurement is determined by the number of free hydrogen ions (H+) in the substance.

Why do we need pH?
pH is a measurement used to specify the acidity or basicity of an aqueous solution. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (H+) content.
However, even chemically pure and neutral water contains some hydrogen ions due to the self-dissociation of water.
It is known that at equilibrium under standard conditions (750 mmHg and 25°C), 1 L of pure water contains mol
y
mol
ions, therefore water at standard temperature and pressure (STP) has a pH of 7.
What to do when the pH of our pool is NOT regulated

Learn about high pool pH, consequences and causes of high pH in your pool.
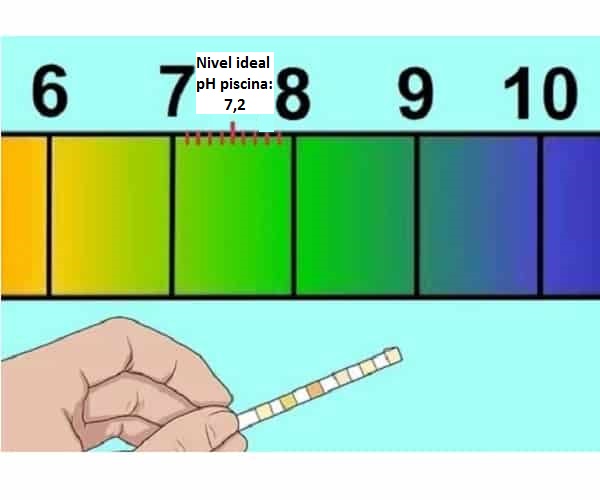
How to raise the pH of the pool and what happens if it is low

How to lower high or alkaline pool pH
Guides on how to maintain the pool in addition to pH: cleaning and disinfecting the water

Useful guide to know how to clean the pool

Guide to maintaining a pool with water in perfect condition
What can the pH of a solution be like?

pH of a solution
pH means “hydrogen potential” or “hydrogen power.” The pH is the negative of the base 10 logarithm of the hydrogen ion activity.
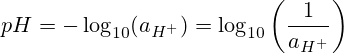
However, in most chemical problems we do not use the activity of hydrogen ions, but rather the molar concentration or molarity.

What are the different pH solutions like?
To start, you should know that the pH scale is logarithmic.
Therefore, it means that the difference by one means a difference by order of magnitude, or ten times and inversely indicates the concentration of hydrogen ions in the solution.
Thus, a lower pH indicates a higher concentration of hydrogen ions and vice versa.

What are acid and base compounds in pH?
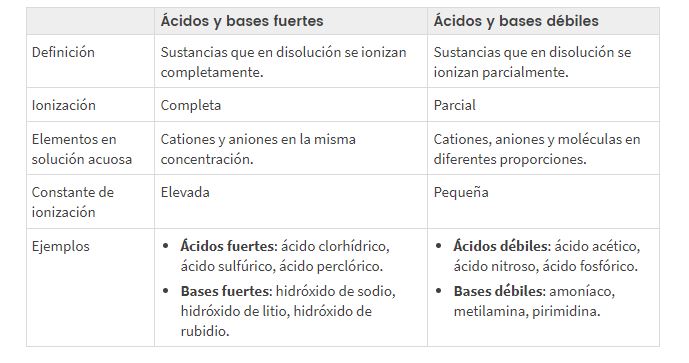
Strong acids and bases are compounds that, for all practical purposes, completely dissociate into their ions in water.
Hence the concentration of hydrogen ions in such solutions can be considered equal to the concentration of the acid.
pH calculation becomes simple
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Calculating pH using molar concentration is different for a strong acid/base and a weak acid/base.
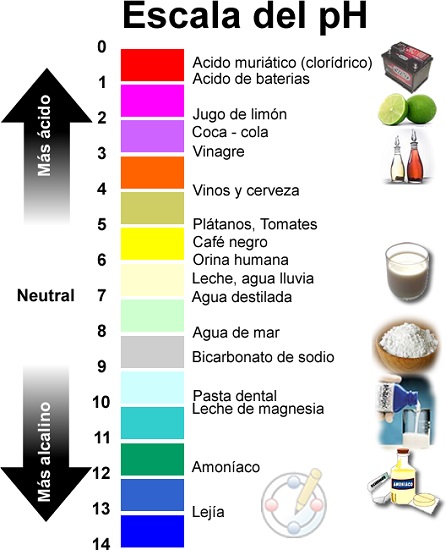
Acidic, neutral and alkaline pH values
Classification of pH Value Scale

What are the pH values?

The pH scale goes from 1 to 14, with pH 7 being the neutral solution.
So, it turns out that pH is a value that is expressed with a logarithmic scale between the values 0 (extremely acidic) and 14 (extremely alkaline); In between we find the value 7 classified as neutral.
pH scale universal ph indicator

What does it mean for a substance to have an acidic or alkaline pH level?
What are acids and bases?
Acids and bases are substances that exist in nature and are distinguished by their pH level, that is, by their degree of acidity or alkalinity. The determination of whether substances are acidic or alkaline is governed by the degree of acidity or alkalinity, measured through the pH scale and goes from 0 (extremely acidic) to 14 (extremely alkaline). Both, however, are usually substances corrosive, often toxic, which nevertheless have numerous industrial and human applications.
How elements are classified based on the pH value scale
Classification of substances as acidic or alkaline according to pH value
Similarly, acidity and alkalinity are two terms that respond to the way of classifying the reaction of any element.

- Likewise, we insist again, The pH scale goes from 1 to 14, with pH 7 being the neutral solution.
- If the pH is less than 7, the solution is overall acidic., the more acidic the lower the pH value, for that reason a acid It is that chemical substance capable of giving up protons (H+) to another chemical substance.
- In return, if the pH is greater than 7, the solution is called basic (or alkaline) and it will be more basic the higher its pH is; and as has been shown basis It is that chemical substance capable of capturing protons (H+) of another chemical substance.
What is alkaline or basic according to the pH scale
What are acidic substances?
- Acidic pH level: pH less than 7
What does it mean when the pH value is acidic?
- That a substance is acidic means that it is rich in H+ (hydrogenions): pH greater than 7
- Hence, Acids are substances with a pH less than 7. (pH of water equal to 7, considered neutral), in whose chemistry large quantities of hydrogen ions commonly appear when water is added. They usually react to other substances by losing protons (H+).
What are neutral substances?
- Neutral pH value: pH equal to 7-
What does it mean that the pH value is neutral?
- pH is a measure of how acidic/basic water is.
- The range is from 0 to 14, with 7 being neutral.
What are alkaline substances?
- Substances with a base or alkaline pH: pH greater than 7.
What does it mean when the pH value is alkaline?
- That a substance is alkaline means that it is poor in H+ (or rich in OH bases-, which neutralize the H+).
- Therefore, Bases, on the other hand, are substances with a pH greater than 7., which in aqueous solutions usually provide hydroxyl ions (OH-) in the middle. They are usually powerful oxidants, that is, they react with protons from the surrounding medium.
What is acidity and alkalinity?
What is acidity and alkalinity in foods?
Next, in the video you will be informed about the endless amount of food that we consume every day but,
- Have you ever wondered why some flavors attract our attention more than others?
- Flavors like salt, bread, soft drinks, juices, even sauces.
- What is this?
- We will explain all this and more to you right now in the recording.
Theories of acid and basic pH

Acid-base theories of pH
What is the Arrhenius ph Theory?

Proposed by the Swede Svante Arrhenius in 1884, constitutes the first modern definition of acids and bases in molecular terms.
Arrhenius acid ph theory
Substance that dissociates in water forming hydrogen cations (H+).
Arrhenius basic ph theory
Substance that dissociates in water forming hydroxide anions (OH-).
ARRHENIUS THEORY What is an acid? What is a base?
Arrhenius acid and basic ph theory video
Brønsted-Lowry pH theory
What is the Brønsted-Lowry theory of pH?

Proposed in 1923 independently by the Dane Johannes Nicolaus Brønsted and English Martin Lowry, is based on the idea of conjugate acid-base pairs.
When an acid, HA, reacts with a base, B, the acid forms its conjugate base, A.-, and the base forms its conjugate acid, HB+, through the exchange of a proton (cation H+):
HA+B⇌A−+HB+
Brønsted-Lowry acid ph theory
Acid ph substance: capable of giving up protons (H+) to a base:
HA+H2O⇌A−+H3O+
Brønsted-Lowry basic ph theory
Substance with basic pH: capable of accepting protons (H+) of an acid:
B+H2O⇌HB++OH−
This theory is considered a generalization of the theory of Arrhenius.
BRÖNSTED-LOWRY THEORY What is an acid? What is a base?
BRÖNSTED-LOWRY pH theory video
Operational definitions of possible pH measurements

What is ACIDITY and ALKALINITY?
What does acidic and basic pH mean?

acid pH
- Firstly, we can find a solution with an acidic pH: a substance that turns the litmus paper blue, reacts with some metals, producing a salt and releasing hydrogen (exothermic reaction).
- Furthermore, substances with acidic pH have a value between 0 and 7.
Basic ph value

- Secondly, there are Base pH: substance that turns red litmus paper blue and that when reacted with phenolphthalein turns pink.
- On the other hand, indicate that they have a pH value between 7 and 14.
Neutral pH

- Finally, the substance with a neutral pH measurement is one that does not react with acid-base indicators.
- Likewise, the pH of these substances is equal to 7.
Substances with strong acidic pH


Measurements of acid solutions on pH
What are acidic pH values like?
- Acids release hydrogen ions, so their aqueous solutions contain more hydrogen ions than neutral water and are considered acidic with a pH less than 7.
What are the most common strong acid pH products?
There are only seven common strong acids:
- – hydrochloric acid HCl
- – nitric acid HNO3
- – sulfuric acid H2SO4
- – hydrobromic acid HBr
- – HI hydroiodic acid
- – perchloric acid HClO4
- – chloric acid HClO3

Strong acid pH formula
Strong acid ph formula
Strong acid pH formula: [HNO3] = [H3O+], and pH = -log[H3O+].
Calculate ph online strong acid
Calculate the pH of a strong acid solution
Substances with strong basic pH

Measurements of basic solutions in pH

What are the acid values in the pH?
Characteristic substances with base pH
- Bases accept hydrogen ions (they bind to some of the hydrogen ions formed by the dissociation of water), so their aqueous solutions contain fewer hydrogen ions than neutral water and are considered basic with a pH greater than 7.

Formula to calculate strong basic ph
Strong acid ph formula
Strong acid pH formula: [HNO3] = [H3O+], and pH = -log[H3O+].
What are the most common strong acid pH products?
There aren't many strong bases either, and some of them are not very soluble in water. Those that are soluble are

- – sodium hydroxide NaOH
- – potassium hydroxide KOH
- – lithium hydroxide LiOH
- – rubidium hydroxide RbOH
- – cesium hydroxide CsOH
Strong base pH calculation
Strong base pH calculation
Substances and formulas with weakly acidic or basic pH

What are weak acid/base pH values like?
The main characteristic of weak acids and bases is that they are partially dissociated in water. A balance is established between the direct and reverse processes, reaching a steady state in which the degree of dissociation depends on the strength of the acid or base.

Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a little more complicated.

Formula for weak acidic pH
Weak acid ph formula
The pH equation remains the same: , but you have to use the acid dissociation constant (Ka) to find [H+].
The formula for Ka is:
where: – concentration of H+ ions
– concentration of conjugated base ions
– concentration of undissociated acid molecules
for a reaction
Calculate pH of a weak acid solution
Calculate pH of a weak acid solution
weak base ph formula
Formula to remove the pH of a weak base
How is the pH of a weak base calculated?
After acquiring pOH from the pOH formula above, the pH can be calculate using the formula pH = pKw – pOH where pK w = 14.00.
Divergences between what the pH value and the pOH are
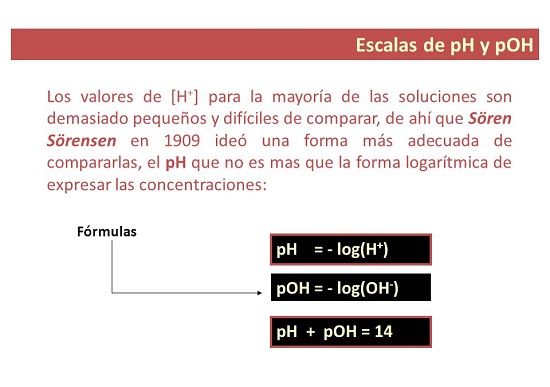
What is the normal pH value?
- In some ways, pH is a measure that It is used to establish the level of acidity or alkalinity of a solution. The “p” is for “potential”, that is why the pH is called: hydrogen potential.
What is the pOH value?
- For his part. pOH is a measure of the concentration of hydroxyl ions in a solution. It is expressed as the negative base 10 logarithm of the concentration of hydroxyl ions and, unlike pH, is used to measure the level of alkalinity of a solution.
Calculate weak base ph
weak base ph calculation
Relative strength of acids and bases

Distinction between strong and weak acidic and basic pH
What does the categorization of strong and weak acid and basic pH depend on?
Depending on how ionized or dissociated an acid or base is, we distinguish between strong and weak acids/bases, terms that describe the ease for drive la electricity (thanks to the greater or lesser presence of ions in the solution).
WEAK AND STRONG ACIDS AND BASES classification, degree of dissociation and pH Examples
Weak and strong acidic and basic pH classification
Degree of ionization of acid and basic pH
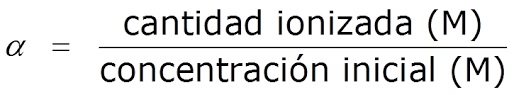
What is the degree of ionization or dissociation of acid and basic pH?
Also called degree of dissociation, α, is defined as the quotient between the amount of ionized acid/base and the amount of initial acid/base:
ááα=amount of ionized acid/base/amount of initial acid/base
It is usually expressed as a percentage (%).
What does the degree of ionization or dissociation of the acid and basic pH mean?
Strong acids and bases
Completely ionized (α≈1). They conduct electricity well.
- Acids: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (1st ionization) and HNO3.
- Bases: Hydroxides of alkali and alkaline earth metals.
Weak acids and bases
Partially ionized: α<1. They conduct electricity poorly.
- Acids: HF(aq), H2S(ac), H2CO3, H2SO3, H3PO4,HNO2 and organic acids, such as CH3COOH.
- Bases: NH3 (or NH4OH) and nitrogenous organic bases, such as amines.
Dissociation constant pH acids and bases
What is the dissociation constant of basic and acidic pH?
It is a measure of the force a acid/base in solution:
| ACID | BASE | |
|---|---|---|
| BALANCE | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| CONSTANT | Ka=[A−][H3O+][HA] | Kb=[HB+][OH−][B] |
| COLOGARITHM | pKa=−logKa | pKb=−logKb |
Relative strength of acidic and basic pH
Acid and basic pH constant
Ionic balance of water

Source: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

What are amphoteres?
Amphoteres what are they
In chemistry, an amphoteric substance is one that can react as either an acid or a base.
Where does the word come from? amphoteric
The word derives from the Greek prefix amphi- (αμφu-), meaning 'both'. Many metals (such as zinc, tin, lead, aluminum, and beryllium) and most metalloids have oxides or hydroxides amphoteric.

Water is an amphiprotic substance
What does it mean that water is an amphiprotic substance?
El water is a substance amphiprotic (can either donate or accept a proton H+), which allows it to act as either an acid or a base (amphotericism).
Ionic balance formula of water

El ionic balance of water refers to the chemical reaction in which two water molecules react to produce an ion oxonium (H3O+) and an ion hydroxide (OH-):
The equilibrium constant, called ionic product of water, and denoted by Kw, can be approximated by the product:
Kw=[H3O+][OH−]
At 25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH and ionic product of water (Kw). ACID-BASE
Acid-base pH indicators

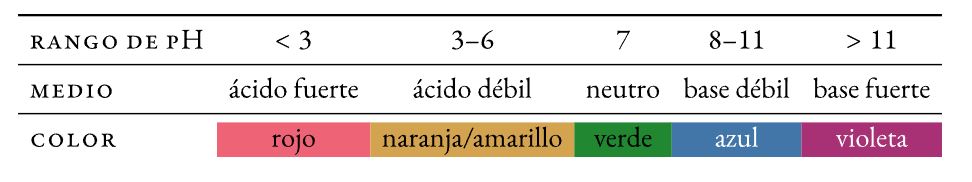
Un indicator pH is a chemical compound halochromic (changes its color -turns— when pH changes) that is added in small quantities to a solution to be able to visually determine its pH (acidity or basicity). The color change is called turn.
Litmus
Water-soluble mixture of different dyes extracted from lichens. Absorbed on filter paper, it constitutes one of the oldest pH indicators used (∼1300).

Methyl orange
Dye azo derivative that turns from red to orange-yellow in acidic medium:

Phenolphthalein
Colorless pH indicator in acidic medium that turns pink in basic medium:

universal indicator
Mix of indicators (thymol blue, methyl red, bromothymol blue and phenolphthalein) which exhibits gentle color changes over a wide range of pH values.
Acid-base neutralization volumetrics

Acid-base titration/titration is a method of quantitative chemical analysis
What is the chemical analysis method of pH acid and basium titration?
Una acid-base titration/titration is a method of quantitative chemical analysis to determine the concentration of an identified acid or base (analyte), neutralizing it exactly with a standard solution of base or acid of known concentration (valorant).

Titration/titration curve of 25 mL of 0.1 M acetic acid with 0.1 M sodium hydroxide.
Neutralization: reaction between a mixture of an acid and a base

What happens if you mix an acid and a base?
The reaction between an acid and a base is called neutralization.
- Neutralization reactions are generally exothermic. which means which They release energy in the form of heat.
- Se They are usually called neutralization because when a acid and with a basis,
- Therefore, the reaction between acids and bases is called neutralization. and more or less eliminates the acidic or basic properties of both compounds, that is, they neutralize each other's properties. producing water and a salt instead.
The mixture of acid and base neutralizes itself the pH does not have to become neutral
- The reason that the mixture of acid and base neutralizes itself the pH does not have to become neutral was sustained because it is by the amount of acid and/or base by which the pH is finally determined.
- Instead, If the amount of H+ and oh- is the same, the solution becomes neutral because they react with each other to form water (H+ + OH- →H20).
Depending on the character of the reacting acid and base, four cases are distinguished:
- Initially, strong acid + strong base
- weak acid + strong base
- Strong acid + weak base
- And lastly, weak acid + weak base
What is an acid and basic pH neutralization reaction?
In a reaction of neutralization, an acid and a base react in a way irreversible to produce a salt and water:
ACID + BASE ⟶ SALT + WATER
Depending on whether the titrant is a strong acid or base, the pH at the equivalence point will be:
| ANALYTE/VALORANT | Strong/Strong | Weak acid/Strong base | Weak base/Strong acid |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| INDICATOR (turns in the middle) | Neutral Stainless - Steel | Basic | Acid |
How to calculate the pH of a solution

What is the pH formula?
In the scientific field, pH is the measurement of the ions within a solution. You may have to calculate the pH based on the concentration.
Formula for calculating ph
Calculate pH using the pH equation: pH = -log[H3O+].
pH calculator for swimming pools
Video to calculate ph of a solution
In 1909, the Danish biochemist Soren Sorensen proposed the term pH to indicate the "hydrogen ion potential." He defined pH as the logarithm of [H+] changed sign. Redefining based on [H3O+].
Solution pH Calculator

pH solution calculator
Calculate the pH of a solution
Below you will find two calculators that you can use to check answers to chemistry problems.
- The first calculates the pH of a solution strong acid o strong foundation.
- And, the second calculates the pH of a solution weak acid o weak base.
Calculate the pH of a strong acid/base solution
Strong Acid/Base Solution pH Calculator
[planetcalc cid=»8830″ language=»es» code=»» label=»PLANETCALC, The pH of a strong acid/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, #c25004″ v=»4165″]
Calculate the pH of a weak acid/base solution
Weak Acid/Base Solution pH Calculator
[planetcalc cid=»8834″ language=»es» code=»» label=»PLANETCALC, The pH of a weak acid/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, #c25004″ v=»4165″]