
Fihirisar abubuwan da ke cikin shafi
En Ok Pool Reform ciki jagoran kula da ruwan tafkin Muna son gabatar muku da labarin mai zuwa: Yadda ake auna alkalinity ruwan tafkin.
pool alkalinity menene

Pool Alkalinity: ainihin siga a cikin lalata ruwan tafkin
Da farko, haskaka wannan Ɗaya daga cikin mahimman sigogi don sarrafawa lokacin da muke aiwatar da kulawa shine alkalinity tare da pH na tafkin.
Yadda za a gudanar da ingantaccen magani na sinadarai na ruwan tafkin
Alkalinity shine ma'auni na abubuwan buffering na ruwa.
Ana auna shi a milligrams na calcium carbonate kowace lita (mg/L) kuma yawanci yana cikin kewayon 80-120 mg/l.
Alkalinity yana da tabbataccen tasiri akan pH saboda yana aiki azaman tafki don ions hydrogen waɗanda zasu iya kawar da acid kuma suyi canji a cikin pH ƙasa da ƙasa.
Sabili da haka, ƙimar alkalinity na 80-120 mg/L yana tabbatar da cewa pH zai ɗan tsaya tsayin daka ko da sunadarai na ruwa ya canza.
Bugu da ƙari, alkalinity yana taka rawa wajen lalata karafa, yana aiki azaman shingen danshi wanda ke kare saman ƙarfe daga lalacewa.
Don haka, isassun ƙimar alkalinity yana da mahimmanci ga masu amfani da ruwa na zama da na kasuwanci.
Menene alkalinity pool
Da farko, bayyana cewa alkalinity shi ne ikon ruwa don neutralize acid, ma'auni na duk abubuwan alkaline da aka narkar da su a cikin ruwa (carbonates, bicarbonates da hydroxides), kodayake borates, silicates, nitrates da phosphates na iya kasancewa.
Alkalinity yana aiki kamar daidaita tasirin canjin pH.
Don haka, idan ba ku jagoranci dabi'un da suka dace ba, ba za ku iya samun ruwa a cikin tafkin ku ba wanda ke da kyau kuma yana bayyana.
Shawarar matakin alkalinity na tafkin
pool alkalinity shawarar shine tsakanin 125-150 ppm.
Tunatarwa: a wasu lokuta, ruwa na iya samun daidaitaccen pH, amma a maimakon haka alkalinity na iya zama ƙasa ko babba.
Yadda ake haɗa pH na ruwan tafkin da alkalinity

Menene pH na tafkin
Haɓaka dabi'a a cikin pH: asarar carbon dioxide
An ayyana pH na bayani azaman mummunan logarithm na ƙimar matsakaicin taro na ions hydrogen.
- Tun da H ions na iya rabuwa cikin H2O da H2CO3, ana iya canza pH ta hanyoyi biyu: ƙara ko cire H2O ko ƙara ko cire H2CO3. Lokacin da carbon dioxide ya ɓace daga wurin tafki ta hanyar ƙaya, pH yana ƙaruwa.
- Wannan saboda H2CO3 yana da acidity mafi girma fiye da H2O; Dangane da daidaiton acid, Kw na H2CO3 shine 3400 idan aka kwatanta da KW na H2O na 25.
- Dangane da dokar Henry, K a don CO2 shine 3,18. Yayin da pH ke ƙaruwa, ƙaddamarwar ions H yana ƙaruwa, kuma yawan protons zai "ionize" zuwa H2O da H2CO3.
Sabili da haka, a cikin tafkin acid, ƙimar canji a cikin pH yana iyakance ta ƙarshe ta ƙimar amsawa tsakanin H2CO3 da H2O.
- ; wannan gudun ya dogara da yanayin zafi, da kuma kasancewar masu hanawa irin su calcium sulfate ko bicarbonate.
- Sabili da haka, yana da mahimmanci don sarrafa pH tare da sauran magungunan tafkin, maimakon amfani da hanyoyin sarrafa pH na al'ada tare da ƙayyadaddun ƙimar manufa.

Wannan zane yana nuna yadda ake cire carbon dioxide (CO2) daga ruwa lokacin da aka hura iska.
- Lokacin da ruwa ya yi iska, carbon dioxide da ke narkar da shi a cikin ruwa ya fara narkewa a cikin ruwa ta dabi'a.
- Yawan iskar carbon dioxide yana tashi zuwa saman tafkin, inda za'a iya kama shi kuma a sake shi cikin yanayi.
Mafi sanyin tafkin, da sauri CO2 zai fito daga cikin ruwa ta halitta.
- A cikin yanayi mai zafi, na rana tare da yawan ƙazanta, yana iya zama ma dole a shayar da ruwa sau da yawa a rana don kiyaye matakan carbon dioxide a cikin iyakar da ake so.
Jadawalin tsarin ma'aunin CO2,

CO2 a dabi'ance yana son neman daidaito tsakanin saman ruwa da iskar yanayi.
Sabili da haka, an saki CO2 har sai ya kasance cikin ma'auni na dangi tare da iska a sama da tafkin. An san wannan al'amari da dokar Henry.
CO2 a dabi'ance yana son neman daidaito tsakanin saman ruwa da iskar yanayi.
Sabili da haka, an saki CO2 har sai ya kasance cikin ma'auni na dangi tare da iska a sama da tafkin. An san wannan al'amari da dokar Henry.
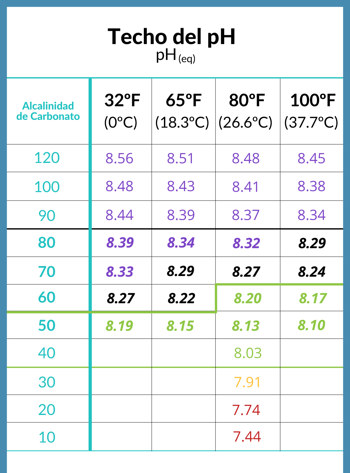
Haɗin kai tsakanin rufin matakin pH na ruwan tafkin da alkalinity
Babban pH pool ruwa da daidaituwa tare da alkalinity
- A cikin tsarin ruwa, pH yana da babban tasiri akan sinadarai na ruwa.
- pH yana sarrafa ƙaddamar da ions daban-daban, kuma canje-canje a cikin pH na iya rinjayar nau'o'in da adadin nau'in da ke nan.
- Alal misali, pH na 7 ya dace don kiyaye aikin tsarin halittu, amma pH na 8 na iya zama ƙasa da ƙasa ga wasu kwayoyin halitta kuma ya yi girma ga sauran nau'in.
Lokacin da CO2 a cikin ruwa ya kai daidaito tare da iska sama da saman ruwa, an ce pH ya kai rufin sa, kuma wannan rufi yana ƙaddara ta matakin alkalinity na carbonate a cikin ruwa.
- Tsarin pH, ko ƙimar pH wanda ya dace da ruwa gaba ɗaya, an ƙaddara shi ta hanyar alkalinity na carbonate na ruwa.
- Za a iya ganin rufin rufi daban-daban a ƙarƙashin yanayi daban-daban a cikin tebur mai zuwa wanda masanin kimiyya Richard Falk ya bayar.
Yaya alkalinity na tafkin da pH na ruwa ya bambanta?
Bambanci Tsakanin Alkalinity Pool da Ruwan pH
Shin kun taɓa mamakin menene bambanci tsakanin pH da alkalinity?
Lokacin da aka yi la'akari da matakin alkalinity ya zama babba
A daya hannun, lokacin da calcium carbonate maida hankali ne sama da 175 ppm, muna magana akan babban alkalinity.
Babban alkalinity yana tasiri
Na gaba, mun ambaci wasu tasirin da ake samarwa lokacin da alkalinity ya girma.
- Mahimmin haɓaka a cikin pH.
- Ruwan da ba shi da gaskiya, ga alama ruwa mai hazo.
- Haushin idanu, kunnuwa, hanci da makogwaro.
- Samar da ma'auni a kan ganuwar da kayan haɗi.
- Hanzarta lalacewa na kayan tafkin.
- Asarar tasiri na maganin tafki.
Menene babban alkalinity?
Yunƙurin alkalinity na iya zama saboda dalilai daban-daban. Sun bambanta da su:
- Ragewar ruwa saboda canje-canje a cikin ƙarar ruwa saboda aikin rana da iska na iya haifar da haɓakar alkalinity.
- Alkalinity yakan karu ta hanyar amfani da tafkin, saboda tasirin man shafawa na rana, gumi da sharar gida...
- Wani lokaci idan muka cika ruwa, idan ya kasance yana hulɗa da dutsen carbonate zai iya samun babban tafkin alkalinity.
- Rashin amfani da sinadarai.
- Rashin aiki a cikin tsarin tace ruwa.
Yadda za a rage yawan alkalinity
Yadda ake Rage Alkadin Ruwa
- Na farko, dole ne mu kashe pool famfo da kuma jira kamar sa'a daya.
- Bayan haka, ana buƙatar ƙara (bisa ga dacewa) adadin da ake buƙata na mai rage pH da rarraba shi don canza shi zuwa carbon dioxide mai bicarbonate. NOTE: Don rage 10 ppm na pool Alkalinity, wajibi ne a rarraba game da 30 ml ga kowane cubic mita na tafkin ruwa (ko dai a cikin ruwa ko m tsari).
- Sa'an nan, bayan sa'a daya, mu mayar da famfo da baya.
- Bayan kamar sa'o'i 24, za mu sake auna matakan alkalinity.
- A gefe guda, idan muka lura cewa matakan alkalinity na tafkin ba su ragu a cikin kwanaki 2 ko 3 ba, za mu sake maimaita tsarin (wani lokaci yana iya zama tsari mai tsada).
- Bugu da ƙari, a kowane lokaci dole ne mu sake nazarin matakan pH, kamar yadda waɗannan zasu iya saukewa.
[akwatin amazon= "B00PQLLPD4" button_text="Sayi"]
Lokacin da aka yi la'akari da matakin alkalinity ya zama ƙasa
A wannan yanayin, lokacin da ƙwayar calcium carbonate ta kasance ƙasa da 125 ppm, muna magana akan ƙaramin alkalinity.
Karancin Sakamakon Alkalinity
Daga cikin tasirin da raguwar alkalinity ke haifarwa a cikin ruwa zamu iya samun:
- Gabaɗaya, pH na tafkin mu zai zama ƙasa. Bugu da ƙari, zai zama da wuya a sarrafa da kuma daidaita shi.
- Saboda waɗannan yanayi, za mu sha maganin kashe ƙwayoyin cuta da yawa tunda ba shi da inganci iri ɗaya.
- Yawan wuce gona da iri na tsarin tacewa.
- Ruwan da ke cikin tafkin mu zai yi kama da kore.
- Yana haifar da lalata da tabo a kan sassan ƙarfe da kayan haɗi na tafkin.
- Har ila yau, yana haifar da hangula na idanu, hanci, makogwaro da fata.
- A ƙarshe, idan kun haɗu da ƙananan alkalinity tare da ƙananan pH, algae za su kasance a cikin ruwa, yana sa ya zama kore.
Menene ke haifar da ƙarancin alkalinity?
Digowar da ba zato ba tsammani a matakin alkalinity a cikin ruwan tafkin na iya zama saboda dalilai masu zuwa:
- Samfuran da ba su dace ba lokacin yin aikin kula da tafkin (kauce wa amfani da allunan tare da ayyuka masu yawa, ruwan ya zama acidic).
- Wani abu mai yiwuwa shi ne kayan aikin tacewa na tafkin ba sa aiki yadda ya kamata.
- Idan akwai sauye-sauyen yanayi mai ƙarfi a cikin zafin jiki.
tada pool alkalinity
Yadda za a tada alkalinity pool

Yadda ake Kara Ruwa Alkalinity
inganta alkalinity
ƙara pool alkalinity: wannan shi ne mafi yawan al'amarin
Wannan shine lamarin da ya fi dacewa, tun da alkalinity na ruwan famfo yawanci yana da ƙasa sosai (a wurare da yawa na Spain yana da ƙasa da 10 ko 20 ppm). Kuma saboda mafi yawan gyaran gyare-gyare na pH mai tsarawa shine rage pH, wanda ke tasowa da chlorine, kuma don rage pH ɗin munyi amfani da acid, wanda kuma yana rage alkalinity (ko da yake ya fi ƙasa da pH). .
Ƙara alkalinity na ruwan tafkin ku na iya zama ɗaya daga cikin matakan farko na dawo da shi cikin ma'auni.
- Lokacin da ruwan ku yana da ƙananan pH, zai iya rinjayar pH na tafkin ku kuma ya haifar da matsaloli da yawa, ciki har da ruwa mai hadari da rashin tsabta. Don taimakawa ƙara yawan alkalinity na ruwan ku, za ku iya amfani da soda foda ko yin burodin soda lu'ulu'u. Tabbatar amfani da adadin da aka ba da shawarar don tafkin ko wurin shakatawa, saboda da yawa zai iya yin mummunan tasiri akan pH na ruwa. Lokacin da kuka fara ganin ci gaba a cikin tsabtar ruwan ku, kuna buƙatar ci gaba da lura da matakan alkalinity don tabbatar da sun tsaya a inda suke buƙata.
tada alkalinity bicarbonate pool
Don haɓaka alkalinity yana da kyau a yi amfani da soda burodi.
Ƙara alkalinity na ruwan tafkin ku na iya zama ɗaya daga cikin matakan farko na dawo da shi cikin ma'auni. Lokacin da ruwan ku yana da ƙananan pH, zai iya rinjayar pH na tafkin ku kuma ya haifar da matsaloli da yawa, ciki har da ruwa mai hadari da rashin tsabta. Don taimakawa ƙara yawan alkalinity na ruwan ku, za ku iya amfani da soda foda ko yin burodin soda lu'ulu'u. Tabbatar amfani da adadin da aka ba da shawarar don tafkin ko wurin shakatawa, saboda da yawa zai iya yin mummunan tasiri akan pH na ruwa. Lokacin da kuka fara ganin ci gaba a cikin tsabtar ruwan ku, kuna buƙatar ci gaba da lura da matakan alkalinity don tabbatar da sun tsaya a inda suke buƙata.
Sodium bicarbonate farin foda ne, mai sauƙin narkewa a cikin ruwa da kuma sarrafa shi, ba shi da guba musamman kuma baya lalata fata idan an taɓa shi, don haka zai yi sauƙi a yi amfani da shi a zuba a cikin tafkin. Bugu da ƙari, sodium bicarbonate ba ya taimakawa wajen tsufa ko guba na ruwa (a cikin wani labarin za mu yi magana game da abin da ake nufi da ruwa mai tsufa ...).
Hakanan za'a iya amfani da ash soda
, da soda caustic, amma ba mu ba da shawarar shi ba, tun da yake sun tsoma baki da yawa tare da pH, kuma ma'anar ita ce ƙoƙarin tayar da alkalinity tare da mafi ƙarancin tasiri akan pH (domin dukan tsari ya fi sauƙi) .
Don ba ku ra'ayi, don ƙara 10 ppm na alkalinity, tasirin pH dangane da abin da ake amfani da shi shine:
Sodium bicarbonate: pH zai ƙara 0,017
Sodium carbonate: pH zai ƙara 0,32
Caustic soda: pH zai ƙara 0,6
Wannan misali ne na tasirin pH-ƙara wanda alkalinity zai iya samu akan acidity na ruwa. Don ba ku ra'ayi, don ƙara 10 ppm na alkalinity, tasirin pH dangane da abin da ake amfani da shi shine:
Sodium bicarbonate: pH zai ƙara 0,017
Sodium carbonate: pH zai ƙara 0,32
Caustic soda: pH zai ƙara 0,6
Wannan misali ne na tasirin pH-ƙara wanda alkalinity zai iya samu akan acidity na ruwa. Don ba ku ra'ayi, don ƙara 10 ppm na alkalinity, tasirin pH dangane da abin da ake amfani da shi shine:
Soda nawa nake bukata?
Ka'idar babban yatsan hannu shine cewa kuna buƙatar gram 17,3 na soda burodi don haɓaka alkalinity ta 10ppm ga kowane m3 na tafkin ku.
Ko menene iri ɗaya:
Adadi a cikin gram = (Alkadi da ake so - Gaskiyar Alkalinity) x (m3 pool) x 1,73
NOTE: Ka tuna cewa waɗannan lissafin ƙididdiga ne kuma suna iya bambanta daga wannan tafkin zuwa wancan.
Bari mu ba da misali don tafkin 50 m3, kuma matakin alkalinity na yanzu shine 30 ppm. A wannan yanayin za mu so mu isa 100 ppm, don haka muna buƙatar:
(100 - 30) x 50 m3 x 1,73 = 6055 grams na yin burodi soda (6 kg, don tattarawa).
Ta yaya zan sarrafa shi?
Manufar ita ce tafiya kadan da kadan. Akwai ka'idoji na ƙididdiga don iyakar adadin sinadarai da ya kamata ku saka a cikin tafkin kowace rana. A cikin wannan kyakkyawar duniyar, matsakaicin adadin bicarbonate a cikin tafkin 50 m3 zai zama gram 360 kowace rana. Amma mun san cewa sau da yawa ba zai yiwu ba, domin babu lokaci. Tare da ruwan da muke da shi a wurare da yawa, zai ɗauki kusan wata ɗaya don gyara alkalinity. Ko kuma a yanayin cire algae, ba za mu iya ɗaukar dogon lokaci ba.
Sabili da haka, yi ƙoƙari ku tafi kadan kadan, kamar yadda kuke da lokaci, tun da ilmin sunadarai na ruwa yana godiya da cewa canje-canjen suna sannu a hankali.
Don gudanar da bicarbonate, tsoma cikin ruwa, kunna tacewa, kuma rarraba cikin tafkin, kamar yadda yake da kusan dukkanin sunadarai. Kuma barin tacewa don kimanin 4-6 hours.
Ana ba da shawarar kashe mai sarrafa pH yayin yin wannan tsari. Ta hanyar gudanar da sodium bicarbonate, pH zai tashi, amma zai kasance na ɗan lokaci, to zai daidaita.
Ba mu ambaci pH a cikin wannan gabaɗayan aikin ba. Kuma shi ne cewa lokacin da ya zama dole don ƙara alkalinity, za mu mayar da hankali ga kafa manufa matakin, sa'an nan za mu auna da daidaita pH na gaba.
Idan pH yana da girma kafin haɓakar alkalinity, sodium bicarbonate ba zai ɗaga shi sosai ba, wannan babban pH dole ne a gyara bayan alkalinity.
Kuma idan pH ya yi ƙasa, zai ɗan hau kaɗan yayin da alkalinity ke sama, amma mafi kyau jira har sai kun sami alkalinity a matakin da ya dace kafin daidaita shi. Har ila yau tuna cewa tare da ƙananan alkalinity, pH ba a kiyaye shi ba, kuma babban ko ƙananan matakansa na iya zama saboda wannan rashin kariya. Abin da ya sa dole ne ku jira har sai kun sami alkalinity tsakanin 80 zuwa 100 sannan ku auna kuma ku daidaita pH.
rage alkalinity
Ba al'ada ba ne don rage alkalinity. Domin ruwa mai wadata yawanci yana da ƙananan matakin, kuma saboda kullum mai kula da pH dole ne ya rage pH (kuma lokacin yin amfani da acid din akwai raguwa a cikin alkalinity).
Amma akwai lokuta, kamar a cikin wasu ruwan ƙasa, inda wadata ya zo tare da pH mai girma da alkalinity. Ko kuma ya faru cewa an ƙara wasu sinadarai a cikin ruwa ba tare da nuna bambanci ba, suna haifar da rashin daidaituwa mai ƙarfi, ɗaya daga cikinsu shine babban alkalinity.
Don rage alkalinity hanya ta bambanta idan pH yana da girma ko ƙasa:
Rage alkalinity tare da babban pH
Kada kayi ƙoƙarin rage pH saboda zai zama da wahala sosai. Babban alkalinity yana da babban iko don kawar da acid (shine ma'anar alkalinity), kuma kowane acid da muka allura zai sami ɗan tasiri akan pH.
Kuma a cikin waɗannan lokuta, dabarar ta ƙunshi allurar etching (wanda ake kira hydrochloric acid ko salfumán ko muriatic acid) gwargwadon yiwuwa a ƙasan tafkin (tare da bututu, alal misali). Dole ne mu yi amfani da acid hydrochloric kamar yadda ya dace sosai, da fatan 30%.
Idan muka yi allurar acid din, dole ne mu kashe wurin sarrafa najasa, kuma ba ya kunna sai washegari.
Adadin hydrochloric acid a cikin cc kuma a 30% abin da muke buƙata shine:
1,55 x (m3 na tafkin) x (karanta alkalinity na yanzu - matakin alkalinity da ake so)
Tare da misalin mu na tafkin 50 m3, kuma muna ɗauka cewa mun fara daga alkalinity na 180 ppm, don isa alkalinity na 100 ppm muna buƙatar:
1,55 x 50 x (180 – 100) = 6200 cc = 6,2 lita na 30% etching
Kada mu yi ƙoƙarin rage fiye da 40-50 ppm na alkalinity kowace rana. Idan ya cancanta, raba shi zuwa lokuta da yawa.
A cikin sa'o'i 24 muna auna matakin alkalinity da pH, kuma zamu iya samun al'amura 3:
- Alkalinity tsakanin 80 da 120, da pH a cikin kewayon kuma (kimanin kasa da 7,5 don wuraren waha tare da chlorine, da 7,8 don wuraren waha tare da bromine): a wannan yanayin muna da kyau, mun yi, yana da sauƙi.
- Alkalinity har yanzu sama da 120, kuma pH mafi girma ko daidai da 7,2. Za mu iya maimaita hanyar allurar etching, amma saita kanmu burin rage alkalinity daga 10 zuwa 10 ppm. Wannan shi ne saboda pH ya kusan kusa da iyaka, kuma idan muka yi nisa zai ragu zuwa matakin da ba za mu iya daga baya ba.
A zahiri, idan a cikin kowane zaman pH ya faɗi ƙasa da 7,0 bai kamata mu ci gaba ba, kuma dole ne mu yi amfani da hanyar da aka bayyana a ƙasa don rage alkalinity tare da ƙarancin pH. - Alkalinity har yanzu yana da girma, amma pH da ke ƙasa 7,0 - 7,2: kada mu ci gaba, dole ne mu yi amfani da fasaha na rage alkalinity tare da ƙananan pH.
Rage alkalinity tare da ƙananan pH
Lokacin da pH yayi ƙasa kuma alkalinity yana da girma, shine mafi munin yanayin, tun lokacin da ya fi wuya a dawo da ma'auni. Idan muka yi amfani da acid, pH zai ragu da yawa, sa'an nan kuma dole ne mu samar da tushe don daidaita shi, amma za su sa alkalinity ya sake tashi, kuma mu shigar da madauki. Ka tuna cewa pH da alkalinity kusan ana canza su a cikin hanya ɗaya, sabili da haka tuki su a kishiyar shugabanci ba a bayyane yake ba.
Kamar yadda ba za mu iya tayar da pH tare da karuwar pH (saboda alkalinity zai kara karuwa), to, dole ne mu yi amfani da hanyar da aka sani da aeration, wanda aka yi amfani da ruwa zuwa tsarin jiki "injecting" iska don ya rasa iskar gas, musamman carbon dioxide (CO2 ). Ba tare da shiga cikin binciken kimiyya da yawa ba, faɗi haka ta hanyar narkar da CO2 a cikin ruwa pH dinsa yana raguwa, kuma idan muka sami damar cire shi daga ruwan, zamu ƙara shi.
Kun karanta daidai, ta hanyar isar da rijiyar ruwa da muke sarrafa cire CO2 kuma haɓaka pH ɗin sa, ba tare da ƙara kowane sinadarai ba, tsari ne na zahiri.
Akwai hanyoyi da yawa don isar da ruwa, duk abin da zaku iya tunani. Kuna iya karkatar da masu tuƙi don ƙirƙirar ɗan vortex, amma tasirin yana da ƙarami. Kuna iya fantsama duk daren…. Amma abin da ya fi amfani shi ne cewa kuna yin ƙaramin "maɓuɓɓuga": tare da bututun PVC da nau'i-nau'i na gwiwar hannu kuna yin irin raƙuman ruwa; Kuna haɗa ƙarshen ɗaya zuwa na'urar motsa jiki, ɗayan kuma za ku sanya filogi na PVC wanda za ku yi ƙananan ramuka, kamar dai kan mai shawa. Ƙarƙashin gwiwar gwiwar zai iya zama digiri 45 don su "toshe" ruwan kai tsaye a cikin tafkin.
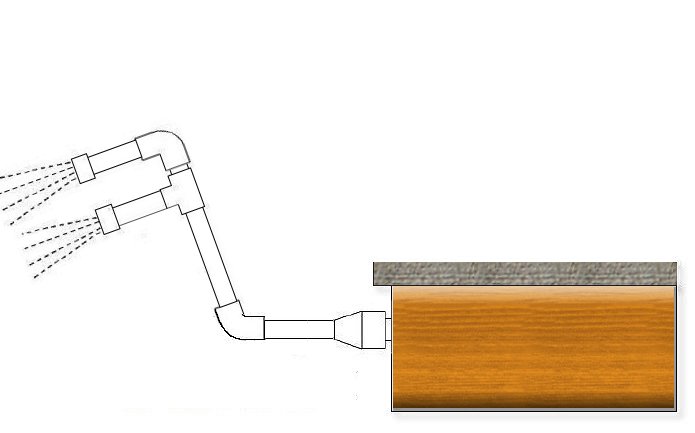
Kuna kunna tacewa, kuma idan za ku iya rufe sauran impellers don matsa lamba ya fi girma, mafi kyau. Ana buƙatar sa'o'i na aiki, ya dogara da girman tafkin da matakin pH, amma za ku yi shi yana gudana ba kasa da sa'o'i 6-8 ba. Kuma za ku ga cewa pH zai tashi kadan kadan.
Hannun hannu da bututu suna da sauƙin samun, wataƙila yana da wahala yadda za a haɗa shi zuwa impeller. Idan masu amfani da tafkin ku sune fararen ABS na yau da kullun tare da hular dunƙule, zaku iya shiga bututun PVC na 32mm tare da ɓangaren mai zuwa:

Da zarar mun gudanar da haɓaka pH zuwa 7,2, za mu sake allurar hydrochloric acid don rage alkalinity. Mafi girma da muka tayar da pH, mafi kyau, tun da za mu iya gyara mafi yawan adadin alkalinity. Idan za mu iya ɗaga shi zuwa 7,6, duk mafi kyau. Ka tuna cewa ba dole ba ne ka yi gyaran alkalinity wanda zai rage pH da ke ƙasa 7,0 - 7,2
MUHIMMIYA SAUKI: Ee, eh, kamar yadda kuka gano, waterfalls, waterfalls, da dai sauransu. a cikin tafkunan ba"m"…. suna da tasiri kai tsaye akan haɓaka pH, don haka amfani da shi (ko cin zarafi) na iya zama haramun dangane da yanayin ...
Sayi Pool alkalinity haɓaka
Pool alkalinity haɓaka farashin
[akwatin amazon= «B071458D86, B07CLBJZ8J, B071458D86, B08TC3DZZD» button_text=»Sayi» ]
Pool ruwa alkalinity mita

Auna don auna alkalinity: tube na nazari.
Don auna jimlar alkalinity na ruwa, zaku iya amfani da madaidaicin tsinkayar nazari (aunawa 4 ko 7 sigogi) waɗanda zasu ba ku damar gano ƙimarsa cikin sauri da sauƙi. Hakanan, zaku iya aiwatar da ma'aunin tare da nau'ikan mita dijital ko ma na'urar daukar hoto.
Sayi samfura don auna alkalinity na tafkin
Yawanci ana auna alkalinity tare da mita pH, wanda ke gano canje-canje a cikin pH a cikin ruwan da ake gwadawa.
Gwajin Alkalinity don wuraren wanka
TSARIN RUWA HOMTIKY 6 IN1 50PCS
Fitowar wannan samfurin tsiri ne na bakin ciki, tare da ƙarshen ɓangarorin ganowa waɗanda aka shirya bisa ga nisan kimiyya, ɗayan ƙarshen don matsayin jagora. Guda ɗaya na gwajin wannan samfur na iya gano abubuwa masu mahimmanci guda shida a lokaci guda a cikin samfurin. A cikin daƙiƙa 30, ana iya gano jimlar taurin, ragowar chlorine kyauta, jimlar chlorine, cyanuric acid, jimlar alkali da pH na ruwan samfurin.
Yadda ake amfani da gwajin alkalinity pool
Sauƙi don amfani da gwajin alkalinity pool
 |  |  |
|---|---|---|
| PH Pool Test StripsAn tsara shi don auna jimlar chlorine, chlorine kyauta, pH, jimlar alkalinity, cyanuric acid da taurin duka. | Bude BottleEach guda 10 na musamman suna cikin marufi na aluminum, an kare shi daga danshi. | Fitar da tsirin gwajin Ciro tsirin gwajin kuma rufe hular kwalbar da kyau bayan amfani. |
 |  |  |
|---|---|---|
| Zuba shi a cikin ruwa Zuba sashin launi na gwajin gwajin a cikin ruwa kuma cire shi bayan dakika 2. | Jira daƙiƙa 30 Ka fitar da tsirin gwajin kuma jira daƙiƙa 30. | Duba Sakamako Kwatanta tsit ɗin gwajin zuwa katin launi a kan kwalabe kuma kammala karatun cikin daƙiƙa 30 don ingantaccen sakamako. |
Bayanin abubuwan ganowa
Jimlar taurin
Jimlar taurin yana nufin adadin calcium da magnesium a cikin ruwa. Jimlar taurin tafkin da ruwan spa ya kamata ya kasance tsakanin 250 zuwa 500 mg/l.
Ragowar chlorine, jimlar chlorine
Chlorine shine mafi yawan maganin kashe kwayoyin cuta a cikin tafkin da kuma ruwan spa, kuma babban manufarsa shine kashe gurɓataccen abu da kuma sanya gurɓataccen abu a cikin ruwa, don haka yana ba da kariya ga masu iyo. Chlorine wanda ke da wuraren tafki mai aiki kuma yana da ikon yin gurɓataccen abu a cikin ruwa ana kiransa ragowar chlorine kyauta. Chlorine wanda ya ƙare ikon kashe shi ta hanyar amsawa tare da gurɓataccen abu ana kiransa chlorine hade. Jimlar chlorine shine jimlar ragowar chlorine da aka ɗaure. Ragowar chlorine na kyauta a cikin tafkin yakamata ya kasance tsakanin 0,3 zuwa 1 mg/L, kuma shawarar ragowar chlorine kyauta a cikin ruwan zafi yakamata ya kasance tsakanin 3 zuwa 5 mg/l.
cyanuric acid
Cyanuric acid, wanda kuma aka sani da "stabilizer" ko "conditioner," yana sa chlorine ya fi kwanciyar hankali lokacin da aka fallasa shi ga hasken ultraviolet na rana. Haɗin chlorine guda biyu (dioxy da trioxy) sun riga sun ƙunshi wasu acid cyanuric. Ci gaba da amfani da kowane ɗayan waɗannan magungunan na iya haɓaka matakin cyanuric acid. Abun cikin acid cyanuric dole ne ya zama ƙasa da ko daidai da 50 MG/L.
NOTE:
Don samun sakamakon gwajin cyanuric acid, pH dole ne ya kasance tsakanin 7.0-8.4 kuma jimlar alkalinity dole ne ya zama ƙasa da ko daidai da 240 mg/L.
total alkali
Jimlar alkalinity shine ma'auni na adadin abubuwan alkaline (musamman bicarbonates da carbonates) a cikin ruwa. Idan ana amfani da sodium chloride, sodium trichloride, ko man shafawa azaman maganin kashe kwayoyin cuta, jimlar alkalinity yakamata ya kasance cikin kewayon 100 zuwa 120 mg/l. Idan ana amfani da calcium, sodium, ko lithium hypooxide azaman maganin kashe kwayoyin cuta, jimlar matakin alkalinity yakamata ya kasance cikin kewayon 80 zuwa 100 mg/l.
PH
pH yana nufin ƙarfin acidic ko alkaline abubuwa a cikin ruwa. pH 7,0 ne tsaka tsaki kuma pH kewayon pool da spa ruwa ya kamata ya kasance tsakanin 7,0 da 7,8.

Bayanan kula:
1. Kada a sanya rigar yatsu a cikin kwalbar.
2. Kada ku taɓa ko gurɓata shingen gwajin gwajin da hannuwanku.
3. Matse hular bayan kowace tsiri na gwaji.
4. Kwatanta launin ɗigon gwaji a cikin haske mai kyau don samun karatu.
5. Ajiye a cikin sanyi, bushe da yanayin duhu.
6. Ana bada shawarar cinyewa a cikin kwanaki 90 bayan buɗewa.
Kariya don amfani da sinadaran reagents:
1. Kada a ƙara reagents na sinadarai lokacin da ake amfani da tafkin.
2. Lokacin da ake ƙara acid, sai a ƙara acid a cikin ruwa, amma kada a ƙara ruwa a cikin acid.
3. Dole ne a yi amfani da duk reagents a hankali kuma a bi umarnin don amfani sosai.
Sayi gwajin alkalinity pool
Pool water alkalinity gwajin tube farashin
Sayi labarin don auna alkalinity na tafkin


