Table of contents of the page
En Ok Pool Reform, in this section within the Swimming pool pH level we will treat the Why is the pH of pool water so important?
Why is the pH of pool water so important?
What is the pH level of the pool and how to control it
What is the pH of the pool for?
The determination of pH is one of the most important and most used analytical procedures in chemistry and biochemistry. pH determines many notable characteristics of the structure and activity of molecules, therefore, the behavior of cells and organisms.
The quality of the water in a pool depends directly on the pH, if it is not maintained it can cause diseases.
The pH is closely related to the quality of the water in swimming pools. This is because chlorine only has an effect if the pH of the pool water is between 6.5 and 8. If the pH of the water is greater than 8 or less than 6.5, no matter how much chlorine is added, it will not act. Therefore, it is important to ensure that the pH is always between 6.5 and 8. This provision is key to ensuring that the pool remains in good condition. A water pH that is too high (greater than 8) produces cloudy water, scale and irritation of the eyes, ears, nose and throat.
Why is it important to control the pH of swimming pools?

Swimming pool hygiene is of great importance, as is maintaining a regular pH that prevents the appearance of microorganisms and guarantees the health of bathers.s.
Using chemical substances in swimming pools is essential for many reasons, including the healthiness of the water and avoiding the appearance of bacteria and microorganisms. However, these additions can unbalance the pH of the water, creating risks for human health. For this reason, the importance of regulating this indicator must be understood, what role other substances such as caustic soda for swimming pools and what strategies to implement to comply with security measures in the operation of these spaces.
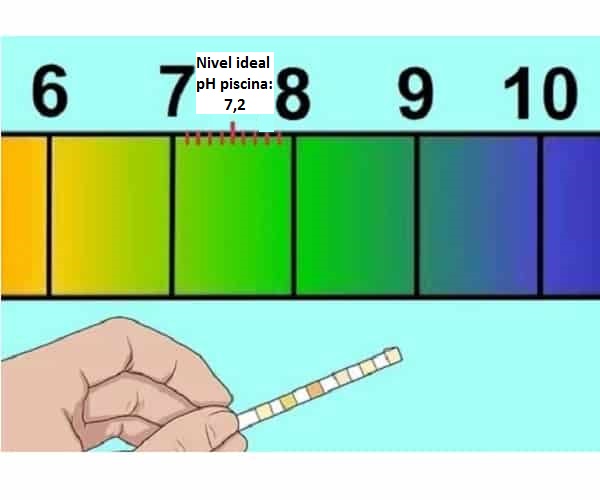
The pH is the alkalinity (acidity) index of water, which should generally be between 7,2 and 7,6. This indicator must be measured in the company of the chlorine index since how suitable the water is for bathing depends on these two factors. For many experts, the ideal value for chlorine in water is 1ppm (parts per million) and alkalinity is 125 or 150 ppm.
The pH of a pool is considered acidic when it is less than 7,2 and alkaline when it is greater than 7,6. The health effects of a bather in a pool with an acidic pH are itching and stinging in the eyes, nose and skin, throat irritation, appearance of eczema, dryness, throat scratching, irritation, among others.

In addition, the acidity of the water can have a direct effect on the wear and tear of the pool facilities, especially on those metal or cement parts that can be corroded by the high level of acidity.
The consequences of having a pH higher than 7,6 will be more noticeable in the water than in the health of bathers. Although people who bathe in an alkaline pool will have symptoms such as dry and irritated skin, the biggest problem will be the poor condition of the water, which will become cloudy, greenish, have a bad smell and facilitate the appearance of microbes, bacteria and algae. . In this way, even if the chlorine level is increased to disinfect it, nothing will be achieved if the pH is not regulated.
Balance of pH values in the human body
Pool ph level

What is the pH of pool water?
What does pool pH mean?

What does pool pH mean?
The pH of the pool that is
What is the pH of the pool: The pH is the hydrogen potential, a value that corresponds to the concentration of hydrogen ions in the water of your pool and consequently it is the coefficient that indicates the degree of acidity or basicity of the water. . Therefore, pH is responsible for indicating the concentration of H+ ions in water, determining its acidic or basic character.
Ideal pH psicin values
The pH scale goes from 1 to 14, with pH 7 being the neutral solution.
pH is a value that is expressed on a logarithmic scale between values 0 and 14.
Therefore, in order to measure the acidity of a liquid, and in our case of pool water, chemicals and now we will use The pH scale includes values from 0 to 14.
ideal pool pH
Pool pH: one of the most significant parameters in pool maintenance.
Appropriate value for pool water pH: between 7.2 and 7.6 ideal neutral pH range.

Thus, Having the pH in this range is not only good for having the water in optimal conditions.s since a reduced or elevated pH considerably decreases the disinfection effect, it is also ideal for the skin and eyes of bathers.
saline pool pH

ph saline pools
- Really, the ph saline pool It is the same as pools treated with chlorine since the maintenance of the Swimmingpool Using salt also requires regular monitoring of the pH of the water.
- Consequently, the pH of salt pools must also have a pH located between 7 and 7,6, the ideal level being between 7,2 and 7,4.
Acidic, neutral and alkaline pH values
Importance of pH in pool maintenance
The power of pH control in pool maintenance
When it comes to pool maintenance, keeping the water chemistry in balance is very important. And as we have explained, the pH level is, in many ways, the basis of that balance. While there are many factors that can affect the pH level, with a little practice and regular attention, you can ensure your water stays in the ideal range for your pool equipment and perfect for your guests.
In this way, pH control is a necessary operation to optimize water quality. and, consequently, to avoid unnecessary expenses by increasing the use of maintenance products, and any risk to your health.
Water, whose formula is H2O (2 hydrogen atoms for one oxygen atom), must be permanently balanced to guarantee you a bath free of inconveniences such as eye and mucous membrane irritations, or the proliferation of algae.
First essential step in water treatment: pH adjustment
As is known, there are many chemical products that are needed to disinfect the pool and ensure the correct treatment of water conservation, which begins by adjusting its PH to the ideal between 7,2 and 7,4.
To carry out a correct control of these levels, we have a series of kits and accessories to analyze the pH of water.
Knowing what the pH of the pool water is is very important for our safety and also to have the pool water in correct condition.
Why it is important to know how to measure pH

Importance of knowing how to measure pH
Definitely, now we will list you many situations in daily life through which a direct implication with pH is derived and in this case it is useful to know how to control and measure the pH since.
Why it is important to learn how to measure pH

- First of all, Chemical reactions in water are affected by the acidity or alkalinity of the solution. This is important not only in the chemistry laboratory, but also in industry, cooking, medicine and in the leisure sector considering the use of swimming pools.
- Basically, pH is carefully regulated in human cells and blood. The normal pH range for blood is between 7,35 and 7,45. Variation of even a tenth of a pH unit can be fatal.
- Soil pH is important for germination and growth of crops. In addition, acid rain caused by natural and man-made pollutants changes the acidity of soil and water, which greatly affects living organisms and other processes.
- To conclude, in cooking, pH changes are used to baking and brewing.
How to measure the pH value and types of meters
How to measure pool pH
How to calculate pH

The pH scale is calculated using a negative logarithm.
The pH value is logarithmic
pH is logarithm of the concentration of H ions+, with the sign changed: Analogously, pOH is defined as the logarithm of the concentration of OH ions-, with the sign changed: The following relationship can be established between the pH and the pOH. Starting from the expression of the ionic product of water (Kw):
Logarithmic pH Formula
- Logarithmic pH Formula: Calculate pH using the pH equation: pH = -log[H3O+].
What does it mean that the pH value is logarithmic?
The fact that pH is logarithmic means that there is a factor of 10 difference between each unit on the scale,
- Therefore, this means that pH 5 is 10 times more acidic than pH 6, and that pH 4 is 100 times more acidic than pH 6.
How to calculate pH with logarithms?
the scale of pH is calculated through a logarithm negative. A logarithm Negative simply indicates how many times a number must be divided. The equation of pH can be written as follows: pH = -log [H3O+]. Sometimes the equation is written like this: pH = -log[H+].
Reason for developing the pH value scale: The pH scale was developed, taking water as a standard.

- It is an experimental fact that only 1 mole in 5,50,000,000 moles of water is ionized into one H+ and one OH-.
- This is the same proportion as one gram of hydrogen ions in 10.000.000 liters of water.
- Therefore, one liter of water contains 1/10.000.000 (or) 1/107 of a gram of H+. For daily use, only the 'Power' figure was used, with the pH symbol placed before it.
Swimming pool ph calculator
Consequences of not having the appropriate pH value
Inadequate pH value is harmful to health
- The first point that we cannot forget is that an inadequate pH value of water can be harmful to our health.
- It is a way to swim safely in a pool without discomfort in the eyes, most of these are caused by pools with high pH, although there is a tendency to believe the burning and other discomfort in the eyes and skin is a consequence of the chlorine in the pool water.
Inadequate pH value means zero water disinfection
- It must be kept in mind that: Without the proper pH balance, the disinfection of the water will be zero, applying disinfectant treatment will be of no use.
Why does the pH in pool water go down or up?
How to raise the pH of the pool and what happens if the level is low
Pool pH consequences and high pH causes
What happens if the pH level is above the recommended value?
High pH pool consequences: What happens if the pH of the pool is high

- Firstly, high pool ph consequences hinder good water circulation and many times, it is a problem that arises from using some types of filters or water heaters.
- The symptoms in our body are dry and irritated skin.
- Likewise, cloudy water changes the pH of the pool, sometimes due to using an insufficient amount of chlorine or an everyday product to disinfect the water.
- As if that were not enough, the high pH will lead to the formation of lime deposits in the pool that will destroy the crystal clear water. These lime deposits will become embedded in the pipes and other installations, affecting their stability and proper functioning. They will also remain attached to walls and floors, altering the appearance and cleanliness of the pool.
Below, if it is of your interest, we provide you with a link to the page where we analyze all the consequences of high pH in swimming pools and its possible causes.