
Table of contents of the page
En Ok Pool Reform, in this section within the Swimming pool pH level we will treat the difference between ph and poh in pool water values.
What is pH in a pool and what should its levels be?

What does ideal pH for swimming pools mean (7,2-7,4)?
The acronym pH means hydrogen potential and is a measure that indicates the acidity or basicity of water.
Then, pH refers to the hydrogen potential, a value that corresponds to the concentration of hydrogen ions in the water of your pool and consequently is the coefficient that indicates the degree of acidity or basicity of the water. Therefore, pH is responsible for indicating the concentration of H+ ions in water, determining its acidic or basic character.
Pool water pH value scale
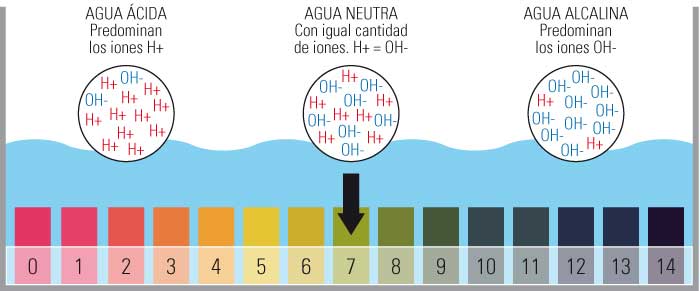

What values does the pool water pH measurement scale include?
- The pH measurement scale includes values from 0 to 14.
- Particularly, 0 being the most acidic, 14 being the most basic and placing the Neutral pH at 7.
- This measurement is determined by the number of free hydrogen ions (H+) in the substance.

Why do we need pH?
pH is a measurement used to specify the acidity or basicity of an aqueous solution. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (H+) content.
However, even chemically pure and neutral water contains some hydrogen ions due to the self-dissociation of water.
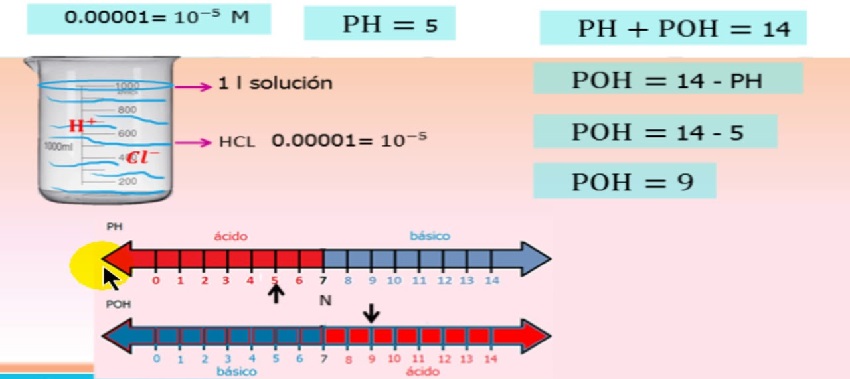
It is known that at equilibrium under standard conditions (750 mmHg and 25°C), 1 L of pure water contains mol
y
mol
ions, therefore water at standard temperature and pressure (STP) has a pH of 7.
What to do when the pH of our pool is NOT regulated
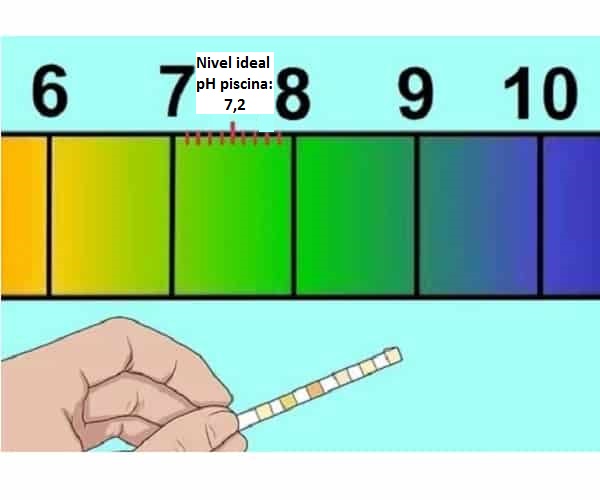
How to raise the pH of the pool and what happens if it is low

How to lower high or alkaline pool pH

5 Effective methods to raise the pH of the pool
Guides on how to maintain the pool in addition to pH: cleaning and disinfecting the water

Useful guide to know how to clean the pool

Guide to maintaining a pool with water in perfect condition
Acidic, neutral and alkaline pH values
Classification of pH Value Scale
What are the pH values?

The pH scale goes from 1 to 14, with pH 7 being the neutral solution.
So, it turns out that pH is a value that is expressed with a logarithmic scale between the values 0 (extremely acidic) and 14 (extremely alkaline); In between we find the value 7 classified as neutral.
pH scale universal ph indicator
What does it mean for a substance to have an acidic or alkaline pH level?
What are acids and bases?
Acids and bases are substances that exist in nature and are distinguished by their pH level, that is, by their degree of acidity or alkalinity. The determination of whether substances are acidic or alkaline is governed by the degree of acidity or alkalinity, measured through the pH scale and goes from 0 (extremely acidic) to 14 (extremely alkaline). Both, however, are usually substances corrosive, often toxic, which nevertheless have numerous industrial and human applications.
What are acidic substances?
- Acidic pH level: pH less than 7
What does it mean when the pH value is acidic?
- That a substance is acidic means that it is rich in H+ (hydrogenions): pH greater than 7
- Hence, Acids are substances with a pH less than 7. (pH of water equal to 7, considered neutral), in whose chemistry large quantities of hydrogen ions commonly appear when water is added. They usually react to other substances by losing protons (H+).
What are neutral substances?
- Neutral pH value: pH equal to 7-
What does it mean that the pH value is neutral?
- pH is a measure of how acidic/basic water is.
- The range is from 0 to 14, with 7 being neutral.
What are alkaline substances?
- Substances with a base or alkaline pH: pH greater than 7.
What does it mean when the pH value is alkaline?
- That a substance is alkaline means that it is poor in H+ (or rich in OH bases-, which neutralize the H+).
- Therefore, Bases, on the other hand, are substances with a pH greater than 7., which in aqueous solutions usually provide hydroxyl ions (OH-) in the middle. They are usually powerful oxidants, that is, they react with protons from the surrounding medium.
Differences between pH and pOH values

How are they related and what are the differences between ph and poh measurements?
Of course, the activity of the ions depends on the concentration of ions and this is described by the equation
pH/poH ion activity equation
where, – hydrogen ion activity
– hydrogen ion activity coefficient
– hydrogen ion concentration
The activity coefficient is a function of ion concentration and approaches 1 as the solution becomes increasingly dilute.
For dilute (ideal) solutions, the standard state of the solute is 1,00 M, so its molarity is equal to its activity.
Therefore, for most problems that assume ideal solutions we can use the base 10 logarithm of the molar concentration, not the activity.
Divergences between what the pH value and the pOH are
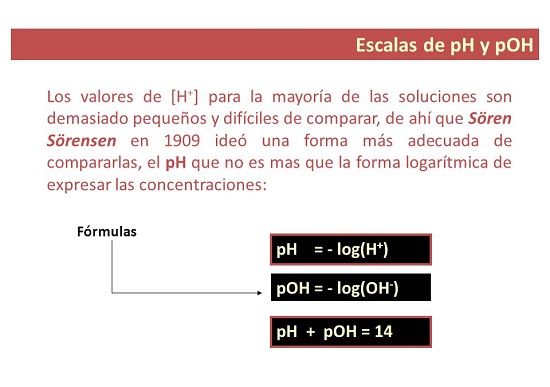
What is the normal pH value?
- In some ways, pH is a measure that It is used to establish the level of acidity or alkalinity of a solution. The “p” is for “potential”, that is why the pH is called: hydrogen potential.
What is the pOH value?
- For his part. pOH is a measure of the concentration of hydroxyl ions in a solution. It is expressed as the negative base 10 logarithm of the concentration of hydroxyl ions and, unlike pH, is used to measure the level of alkalinity of a solution.

How is the pH or pOH value calculated?
What is the formula for ph scale values?
- As is already known, in the scientific field, the pH is the measure de the ions inside de a solution. You may have to calculate pH based on concentration. Calculate the pH Using the equation of pH: pH = -log[H3O+].
What is the formula to calculate pOH?
- Also, the pOH (or OH potential) is a measure of the basicity or alkalinity of a solution. Also se uses pH = – log [H3O+] to measure the concentration of hydronium ions [H3O+].

Key Equations to Calculate the pH or pOH Value
- pH=−log[H3O+]
- pOH=−log[OH−]
- [H3O+] = 10-pH
- [OH-] = 10-pOH
- pH + pOH = pKw = 14.00 at 25 °C.
What is the difference between the pH value scale and the pOH scale?
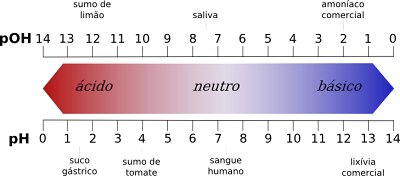
Inequalities between pH scale values
- On the one hand, the pH scale gives acidic values from 1 to 6 while the pOH scale gives acidic values from 8 to 14.
- Conversely, the pH scale gives basic values from 8 to 14, while the pOH scale gives basic values from 1 to 6.
Logarithm scale relationship of pH and pOH with their values
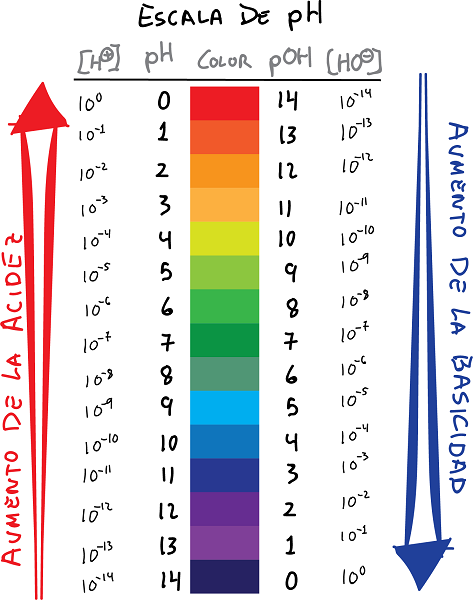
Ph and pOH scale connection with colors and values
- pH is the logarithm of the H ion concentration+, with the sign changed:
- Similarly, it is defined pOH as the logarithm of the concentration of OH ions-, with the sign changed: The following relationship can be established between the pH and pOH.
- Basically, pH values give the negative logarithm of the hydrogen ion concentration, whereas the pOH value gives the negative logarithm of the hydroxide ion concentration.
Difference between the pH and pOH value scale
Discordances between the ph value table and pOH value
After that, we provide you with a film with which you can see that pH measures the concentrations of hydrogen ions, while pOH measures the concentrations of hydroxyl anions or hydroxide ions.


