
Inkomba yokuqukethwe kwekhasi
En Ok Pool Reform, kulesi sigaba ngaphakathi kwe Izinga le-pH lamachibi okubhukuda sizophatha i umehluko phakathi kwe-ph ne-poh kumanani wamanzi wechibi.
Iyini i-pH echibini futhi amazinga ayo kufanele abe kanjani?

Isho ukuthini i-pH ekahle kumachibi okubhukuda (7,2-7,4)
I-acronym pH imele i-hydrogen engaba khona futhi iyisilinganiso esibonisa ubumuncu noma ubuqiniso bamanzi.
Ngakho, I-pH isho amandla e-hydrogen, inani elihambisana nokuhlangana kwama-ion e-hydrogen emanzini echibini lakho futhi ngakho-ke iyi-coefficient ebonisa izinga le-asidi noma isisekelo samanzi. Ngakho-ke, i-pH iphethe ukukhombisa ukugcwala kwama-ion e-H+ emanzini, ukucacisa i-acidic yawo noma uhlamvu oluyisisekelo.
Isikali samanani e-pH amanzi echibi lokubhukuda
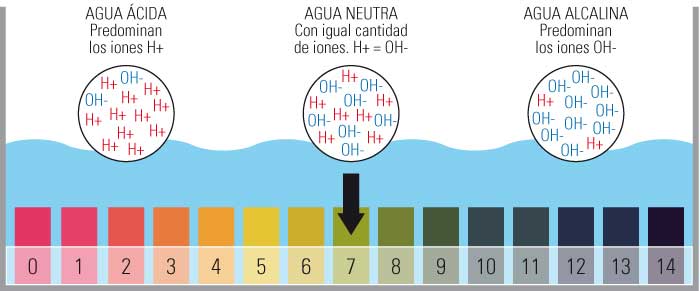

Imaphi amanani afakwa isikali sokulinganisa i-pH yamanzi echibini?
- Isikali sokulinganisa i-pH sihlanganisa amanani asuka ku-0 kuye ku-14.
- Ikakhulukazi u-0 one-acidic kakhulu, u-14 uyisisekelo kakhulu futhi obeka i-pH emaphakathi ku-7.
- Lesi silinganiso sinqunywa inani lama-ion e-hydrogen amahhala (H+) entweni.

Kungani sidinga i-pH?
I-pH isilinganiso esisetshenziselwa ukucacisa ubumuncu noma ubuqiniso besisombululo esinamanzi. Ukuthi isixazululo esinamanzi siphendula njenge-asidi noma isisekelo kuncike ekuqukethe ama-ion e-hydrogen (H+).
Kodwa-ke, ngisho namanzi ahlanzekile ngamakhemikhali futhi angathathi hlangothi aqukethe ama-ion e-hydrogen ngenxa yokuzihlukanisa kwamanzi.
Kuyaziwa ukuthi ekulinganisweni ngaphansi kwezimo ezijwayelekile (750 mmHg kanye no-25°C), i-1 L yamanzi ahlanzekile iqukethe. imvukuzane
y
imvukuzane
ama-ion, ngakho-ke, amanzi asezingeni lokushisa elijwayelekile nokucindezela (STP) ane-pH engu-7.
Okufanele ukwenze lapho i-pH yechibi lethu AYIlawulwa

Yazi imiphumela yechibi eliphezulu le-pH kanye nezimbangela ze-pH ephezulu echibini lakho
Indlela yokukhulisa i-pH yechibi nokuthi kwenzekani uma iphansi

Indlela Yehlisa Ichibi Eliphezulu noma Eline-alkaline pH
Imihlahlandlela yokuthi kwenziwa kanjani ukunakekelwa kwechibi ngaphezu kwe-pH: ukuhlanza amanzi kanye nokubulala amagciwane

Umhlahlandlela owusizo ukwazi ukuhlanza ichibi

Umhlahlandlela wokunakekela ichibi elinamanzi asesimweni esihle
Amanani e-pH ane-Acidic, neutral kanye ne-alkaline
Ukuhlukaniswa Kwesikali Samanani we-pH
Ayini amanani we-pH

Isikali se-pH sisuka ku-1 siye ku-14, kanti i-pH 7 iyisixazululo esingathathi hlangothi.
Ngakho-ke, kuvela ukuthi i-pH iyinani elivezwa esikalini se-logarithmic phakathi kwamanani 0 (okune-asidi ngokwedlulele) kanye no-14 (okune-alkaline ngokwedlulele); Phakathi sithola inani elingu-7 libhalwe njengeliphakathi nendawo.
Inkomba ye-pH yendawo yonke yesikali se-pH
Kusho ukuthini ukuthi into ine-pH ene-asidi noma ene-alkali?
Ayini ama-asidi nezisekelo?
Ama-Acid nezisekelo yizinto ezikhona emvelweni futhi zihlukaniswa ngeleveli yazo ye-pH, okungukuthi, ngezinga le-acidity noma i-alkalinity. Ukunqunywa kokuthi izinto zine-acidic noma i-alkaline kulawulwa yizinga le-asidi noma i-alkalinity elinganiswa ngesikali se-pH futhi isukela ku-0 (ine-acidi ngokwedlulele iye ku-14 (i-alkaline ngokwedlulele). Kokubili, nokho, ngokuvamile kuyizinto ezonakalisayo, ezivamise ukuba nobuthi, obuthi nokho banezicelo eziningi zezimboni nezabantu.
Yiziphi izinto ezine-acidic?
- Izinga le-Acid pH: pH ngaphansi kuka-7
Kusho ukuthini ukuthi inani le-pH line-acidic?
- Ukuthi into ethile ine-acidic kusho ukuthi inothile nge-H+ (ama-hydrogen ions): i-pH enkulu kuno-7
- Ngakho, Ama-Acids yizinto ezine-pH engaphansi kuka-7. (i-pH yamanzi ilingana no-7, ibhekwa njengengathathi hlangothi), ikhemikhali yayo ngokuvamile iqukethe amanani amakhulu e-hydrogen ion lapho wengeza amanzi. Ngokuvamile zisabela nezinye izinto ngokulahlekelwa ama-proton (H+).
Yiziphi izinto ezingathathi hlangothi?
- Inani le-pH emaphakathi: i-pH ilingana no-7-
Kusho ukuthini ukuthi inani le-pH alithathi hlangothi?
- I-pH iyisilinganiso sokuthi amanzi ane-acidic/ayisisekelo kangakanani.
- Ububanzi busuka ku-0 kuye ku-14, kanti u-7 ungathathi hlangothi.
Yiziphi izinto ezine-alkaline?
- Izinto ezine-pH yesisekelo noma ye-alkali: pH enkulu kuno-7.
Kusho ukuthini uma inani le-pH liyi-alkaline?
- Ukuthi into ine-alkaline kusho ukuthi impofu ku-H+ (noma ucebile ngezisekelo ze-OH-, okwenza i-H+).
- Kukho konke lokhu, Izisekelo, ngakolunye uhlangothi, ziyizinto ezine-pH enkulu kuno-7., okuthi ezixazululweni ezinamanzi ngokuvamile anikeze ama-ion hydroxyl (OH-) phakathi. Avame ukuba ama-oxidants anamandla, okungukuthi, asabela ngama-protons avela endaweni ezungezile.
Umehluko phakathi kwamanani we-pH ne-pOH

Zihlobene kanjani futhi yimuphi umehluko phakathi kwezilinganiso ze-ph ne-poh?
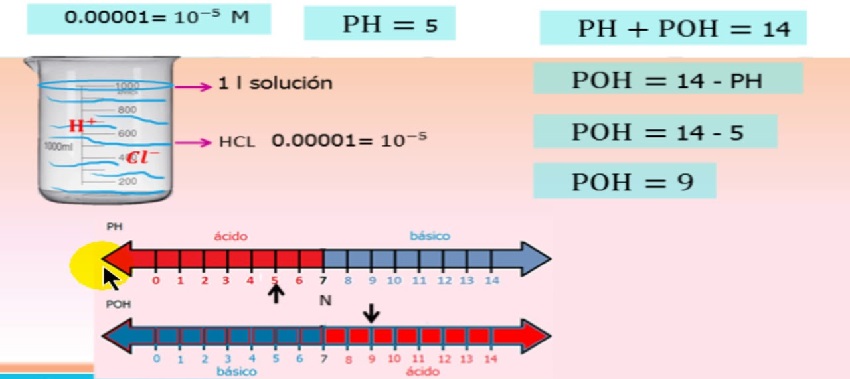
Yiqiniso, umsebenzi we-ion uncike ekugxilweni kwe-ion futhi lokhu kuchazwe ku-equation
Izibalo zomsebenzi we-pH/poH ion
kuphi, - Umsebenzi we-hydrogen ion
- umsebenzi we-coefficient ye-hydrogen ion
- Ukuhlushwa kwe-hydrogen ion
I-coefficient yomsebenzi iwumsebenzi wokugxilisa i-ion futhi isondela ku-1 njengoba isisombululo siya ngokuya sincipha.
Ukuze uthole izixazululo ze-dilute (ezikahle), isimo esijwayelekile se-solute ngu-1,00 M, ngakho-ke i-molarity yayo ilingana nomsebenzi wayo.
Ngakho-ke, ezinkingeni eziningi ezithatha izixazululo ezifanele singasebenzisa i-logarithm kusisekelo esingu-10 sokugxila kwe-molar, hhayi umsebenzi.
Umehluko phakathi kwenani le-pH ne-pOH
Liyini inani le-pH elivamile?
- Ngandlela thile, i-pH iyisilinganiso sokuthi esetshenziselwa ukusungula izinga le-acidity noma i-alkalinity yesisombululo. Igama elithi “p” limele “okungenzeka”, yingakho i-pH ibizwa ngokuthi: amandla e-hydrogen.
Liyini inani le-pOH?
- Ngokwengxenye yakho. I-pOH isilinganiso sokuhlangana kwama-ion e-hydroxyl esixazululweni. Ivezwa njengesisekelo se-logarithm engu-10 ye-negative logarithm ye-hydroxyl ion concentration futhi, ngokungafani ne-pH, isetshenziselwa ukukala izinga le-alkalinity lesisombululo.

Ibalwa kanjani i-pH noma inani le-pOH?
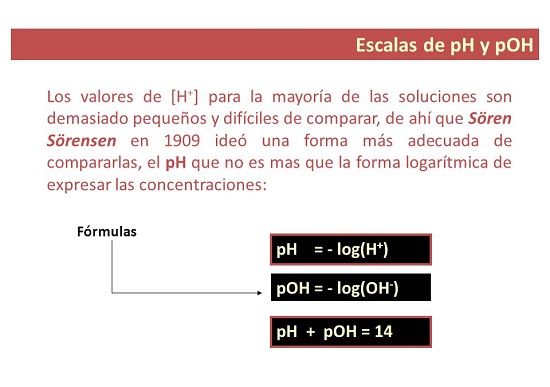
Ithini ifomula yamanani esikali se-ph?
- Njengoba sekwaziwa kakade, emkhakheni wesayensi, i pH yisilinganiso de ama-ion ngaphakathi de isixazululo. Kungase kudingeke wenze kanjalo bala pH ngokusekelwe ekugxiliseni ingqondo. Bala i- pH Ukusebenzisa i-equation ye pH: pH = -log[H3O+].
Ithini ifomula yokubala i-pOH?
- Futhi, i pOH (noma amandla we-OH) isilinganiso sesisekelo noma i-alkalinity yesixazululo. Futhi se isebenzisa i-pH = – log [H3O+] ukukala ukugcwala kwama-ion e-hydronium [H3O+].

Izibalo Ezibalulekile zokubala inani le-pH noma le-pOH
- pH=−log[H3O+]
- pOH=−log[OH−]
- [H3O+= 10-pH
- [oh-= 10-pOH
- pH + pOH =pKw = 14.00 ku-25 °C.
Uyini umehluko phakathi kwesilinganiso samanani we-pH kanye nese-pOH?
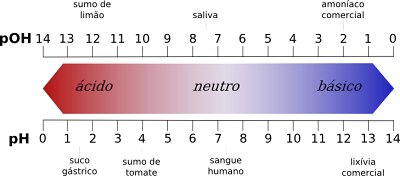
Ukungalingani phakathi kwamanani esikali se-pH
- Ngakolunye uhlangothi, isikali se-pH sinikeza amanani e-asidi ukusuka ku-1 kuye ku-6 ngenkathi isikali se-pOH sinikeza amanani e-asidi ukusuka ku-8 kuye ku-14.
- Ngakolunye uhlangothi, isikali se-pH sinikeza amanani ayisisekelo ukusuka ku-8 kuye ku-14, kuyilapho isikali se-pOH sinikeza amanani ayisisekelo ukusuka ku-1 kuye ku-6.
Ubudlelwano besikali sesilogi se-ph ne-pOH ngamavelu awo
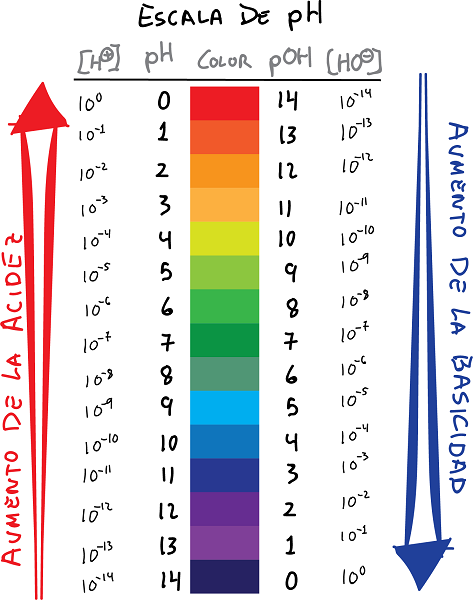
ph kanye nokuxhumeka kwesikali se-pOH ngemibala namanani
- pH i-logarithm yokuhlushwa kwama-H ions+, nophawu olushintshile:
- Ngokufanayo, chaza pOH njenge-logarithm ye-OH ion concentration-, ngophawu olushintshile: Ubudlelwano obulandelayo bungasungulwa phakathi kwe pH futhi i pOH.
- Ngokuyisisekelo, amanani we-pH anikeza i-logarithm eyinegethivu yokugxiliswa kwe-ion ye-hydrogen, kuyilapho inani le-pOH linikeza i-logarithm eyinegethivu yokugxiliswa kwe-ion ye-hydroxide.
Umehluko phakathi kwesikali samanani we-pH kanye ne-pOH
Umehluko phakathi kwethebula lenani elingu-ph kanye nenani le-pOH
Ngemva kwalokho, sikuhlinzeka ngemuvi lapho ungabona khona ukuthi i-pH ikala ukugxiliswa kwama-ion e-hydrogen, kuyilapho i-pOH ikala ukugxiliswa kwama-ionion e-hydroxyl noma ama-ion e-hydroxide.


