En Ok Pool Reform, kulesi sigaba ngaphakathi kwe Izinga le-pH lamachibi okubhukuda Sizobhekana nombuzo olandelayo: Isho ukuthini i-acidic nesisekelo se-pH?
Inkomba yokuqukethwe kwekhasi
Iyini i-pH echibini futhi amazinga ayo kufanele abe kanjani?

Isho ukuthini i-pH ekahle kumachibi okubhukuda (7,2-7,4)
I-acronym pH imele i-hydrogen engaba khona futhi iyisilinganiso esibonisa ubumuncu noma ubuqiniso bamanzi.
Ngakho, I-pH isho amandla e-hydrogen, inani elihambisana nokuhlangana kwama-ion e-hydrogen emanzini echibini lakho futhi ngakho-ke iyi-coefficient ebonisa izinga le-asidi noma isisekelo samanzi. Ngakho-ke, i-pH iphethe ukukhombisa ukugcwala kwama-ion e-H+ emanzini, ukucacisa i-acidic yawo noma uhlamvu oluyisisekelo.
Isikali samanani e-pH amanzi echibi lokubhukuda

Imaphi amanani afakwa isikali sokulinganisa i-pH yamanzi echibini?
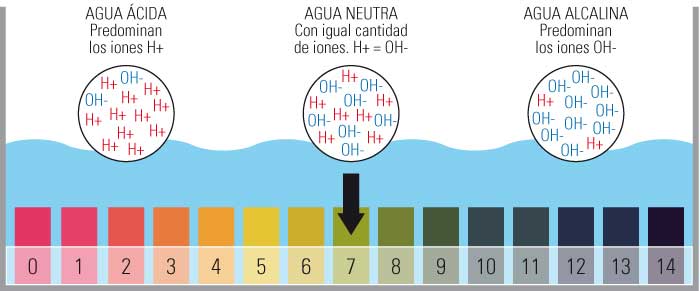
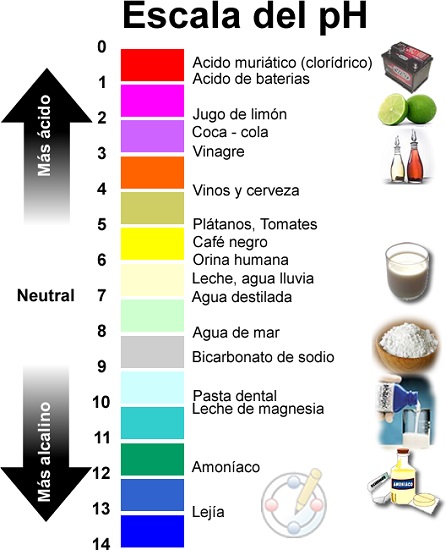
- Isikali sokulinganisa i-pH sihlanganisa amanani asuka ku-0 kuye ku-14.
- Ikakhulukazi u-0 one-acidic kakhulu, u-14 uyisisekelo kakhulu futhi obeka i-pH emaphakathi ku-7.
- Lesi silinganiso sinqunywa inani lama-ion e-hydrogen amahhala (H+) entweni.

Kungani sidinga i-pH?
I-pH isilinganiso esisetshenziselwa ukucacisa ubumuncu noma ubuqiniso besisombululo esinamanzi. Ukuthi isixazululo esinamanzi siphendula njenge-asidi noma isisekelo kuncike ekuqukethe ama-ion e-hydrogen (H+).
Kodwa-ke, ngisho namanzi ahlanzekile ngamakhemikhali futhi angathathi hlangothi aqukethe ama-ion e-hydrogen ngenxa yokuzihlukanisa kwamanzi.
Kuyaziwa ukuthi ekulinganisweni ngaphansi kwezimo ezijwayelekile (750 mmHg kanye no-25°C), i-1 L yamanzi ahlanzekile iqukethe. imvukuzane
y
imvukuzane
ama-ion, ngakho-ke, amanzi asezingeni lokushisa elijwayelekile nokucindezela (STP) ane-pH engu-7.
Okufanele ukwenze lapho i-pH yechibi lethu AYIlawulwa

Yazi imiphumela yechibi eliphezulu le-pH kanye nezimbangela ze-pH ephezulu echibini lakho
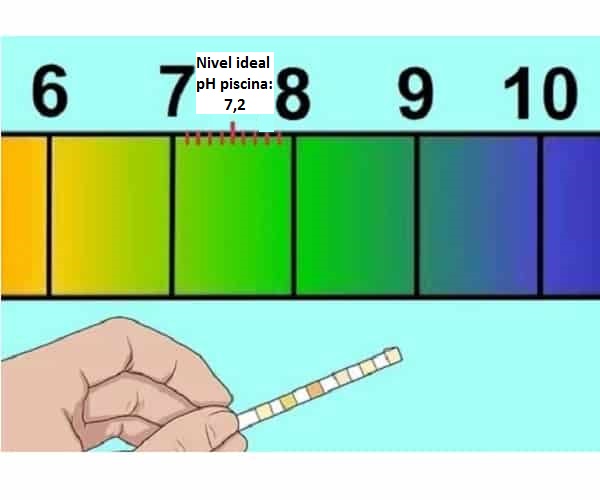
Indlela yokukhulisa i-pH yechibi nokuthi kwenzekani uma iphansi

Indlela Yehlisa Ichibi Eliphezulu noma Eline-alkaline pH
Imihlahlandlela yokuthi kwenziwa kanjani ukunakekelwa kwechibi ngaphezu kwe-pH: ukuhlanza amanzi kanye nokubulala amagciwane

Umhlahlandlela owusizo ukwazi ukuhlanza ichibi

Umhlahlandlela wokunakekela ichibi elinamanzi asesimweni esihle
Ingaba kanjani i-pH yesixazululo?

i-pH yesisombululo
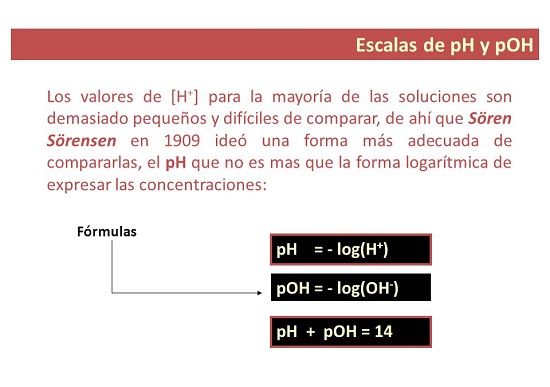
I-pH imele "amandla e-hydrogen" noma "amandla e-hydrogen." I-pH iyinegethivu yesisekelo esingu-10 logarithm somsebenzi we-hydrogen ion.
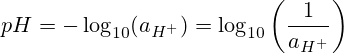
Kodwa-ke, ezinkingeni eziningi zamakhemikhali asisebenzisi umsebenzi we-hydrogen ions, kodwa ukuhlushwa kwe-molar noma i-molarity.

Zinjani izixazululo ezihlukene ze-pH
Okokuqala, kufanele wazi ukuthi isikali se-pH siyi-logarithmic.
Ngakho-ke, kusho ukuthi umehluko ngenye indlela umehluko ngokuhleleka kobukhulu, noma izikhathi eziyishumi futhi ngokuphambene kubonisa ukuhlushwa kwe-hydrogen ions kusixazululo.
Ngakho-ke, i-pH ephansi ibonisa ukuhlushwa okuphezulu kwama-hydrogen ions futhi ngokuphambene nalokho.

Yiziphi i-asidi nesisekelo samakhemikhali ku-pH
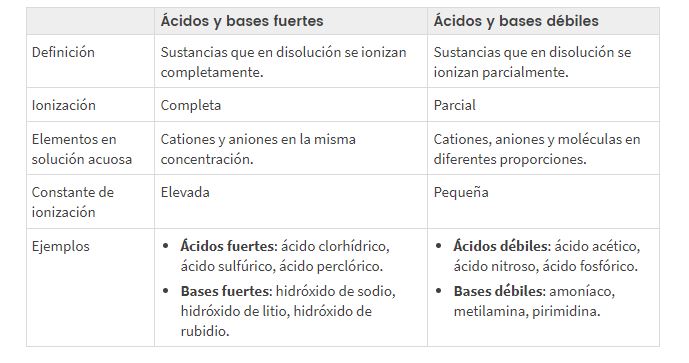
Ama-asidi aqinile nezisekelo eziqinile ziyizinhlanganisela okuthi, ngazo zonke izinhloso ezingokoqobo, zihlukanise ngokuphelele ama-ion awo emanzini.
Ngakho-ke ukugcwala kwama-ion e-hydrogen ezixazululweni ezinjalo kungathathwa njengokulingana nokugcwala kwe-asidi.
Ukubalwa kwe-pH kuba lula
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Ukubalwa kwe-pH kusetshenziswa ukugxiliswa kwe-molar kuhlukile ku-asidi eqinile/isisekelo ne-asidi/isisekelo esibuthakathaka.
Amanani e-pH ane-Acidic, neutral kanye ne-alkaline
Ukuhlukaniswa Kwesikali Samanani we-pH

Ayini amanani we-pH

Isikali se-pH sisuka ku-1 siye ku-14, kanti i-pH 7 iyisixazululo esingathathi hlangothi.
Ngakho-ke, kuvela ukuthi i-pH iyinani elivezwa esikalini se-logarithmic phakathi kwamanani 0 (okune-asidi ngokwedlulele) kanye no-14 (okune-alkaline ngokwedlulele); Phakathi sithola inani elingu-7 libhalwe njengeliphakathi nendawo.
Inkomba ye-pH yendawo yonke yesikali se-pH

Kusho ukuthini ukuthi into ine-pH ene-asidi noma ene-alkali?
Ayini ama-asidi nezisekelo?
Ama-Acid nezisekelo yizinto ezikhona emvelweni futhi zihlukaniswa ngeleveli yazo ye-pH, okungukuthi, ngezinga le-acidity noma i-alkalinity. Ukunqunywa kokuthi izinto zine-acidic noma i-alkaline kulawulwa yizinga le-asidi noma i-alkalinity elinganiswa ngesikali se-pH futhi isukela ku-0 (ine-acidi ngokwedlulele iye ku-14 (i-alkaline ngokwedlulele). Kokubili, nokho, ngokuvamile kuyizinto ezonakalisayo, ezivamise ukuba nobuthi, obuthi nokho banezicelo eziningi zezimboni nezabantu.
Indlela ama-elementi ahlukaniswa ngayo ngokusekelwe esikalini samanani e-pH
Ukuhlukaniswa kwezinto kuma-acids noma ama-alkaline ngokuya ngevelu ye-pH
Ngokufanayo, i-acidity ne-alkalinity amagama amabili aphendula endleleni yokuhlukanisa ukusabela kwanoma iyiphi i-elementi.

- Ngokufanayo, siyagcizelela futhi, Isikali se-pH sisuka ku-1 siye ku-14, kanti i-pH 7 iyisixazululo esingathathi hlangothi.
- Uma i-pH ingaphansi kuka-7, isisombululo sine-acidic., i-asidi eyengeziwe iyancipha inani le-pH ngaleso sizathu a i-asidi yileyo khemikhali ekwazi ukunikela ngama-proton (H+) kwenye ikhemikhali.
- Esikhundleni salokho, uma i-pH ingaphezu kuka-7, ikhambi libizwa ngokuthi isisekelo (noma i-alkaline) futhi izoba yisisekelo kakhulu lapho iphezulu i-pH yayo; futhi njengoba kubonisiwe isisekelo yileyo khemikhali ekwazi ukubamba ama-proton (H+) yenye ikhemikhali.
Yini i-alkaline noma eyisisekelo ngokwesilinganiso se-pH
Yiziphi izinto ezine-acidic?
- Izinga le-Acid pH: pH ngaphansi kuka-7
Kusho ukuthini ukuthi inani le-pH line-acidic?
- Ukuthi into ethile ine-acidic kusho ukuthi inothile nge-H+ (ama-hydrogen ions): i-pH enkulu kuno-7
- Ngakho, Ama-Acids yizinto ezine-pH engaphansi kuka-7. (i-pH yamanzi ilingana no-7, ibhekwa njengengathathi hlangothi), ikhemikhali yayo ngokuvamile iqukethe amanani amakhulu e-hydrogen ion lapho wengeza amanzi. Ngokuvamile zisabela nezinye izinto ngokulahlekelwa ama-proton (H+).
Yiziphi izinto ezingathathi hlangothi?
- Inani le-pH emaphakathi: i-pH ilingana no-7-
Kusho ukuthini ukuthi inani le-pH alithathi hlangothi?
- I-pH iyisilinganiso sokuthi amanzi ane-acidic/ayisisekelo kangakanani.
- Ububanzi busuka ku-0 kuye ku-14, kanti u-7 ungathathi hlangothi.
Yiziphi izinto ezine-alkaline?
- Izinto ezine-pH yesisekelo noma ye-alkali: pH enkulu kuno-7.
Kusho ukuthini uma inani le-pH liyi-alkaline?
- Ukuthi into ine-alkaline kusho ukuthi impofu ku-H+ (noma ucebile ngezisekelo ze-OH-, okwenza i-H+).
- Kukho konke lokhu, Izisekelo, ngakolunye uhlangothi, ziyizinto ezine-pH enkulu kuno-7., okuthi ezixazululweni ezinamanzi ngokuvamile anikeze ama-ion hydroxyl (OH-) phakathi. Avame ukuba ama-oxidants anamandla, okungukuthi, asabela ngama-protons avela endaweni ezungezile.
Iyini i-acidity ne-alkalinity?
Iyini i-acidity kanye ne-alkalinity ekudleni
Ngemuva kwalokho, kuvidiyo uzokwaziswa ngenani elingapheli lokudla esikudla usuku nosuku kodwa,
- Wake wazibuza ukuthi kungani amanye ama-flavour abamba ukunaka kwethu kakhulu kunamanye?
- Ama-flavour afana nosawoti, isinkwa, iziphuzo ezibandayo, amajusi, ngisho namasoso.
- Kungani kunjalo?
- Sizokuchazela konke lokhu nokunye okuningi njengamanje ekurekhodeni.
Imibono ye-acidic ne-pH eyisisekelo

I-Acid-base theory ye-pH
Iyini i-Arrhenius pH Theory?

ehlongozwa isiswedish Svante Arrhenius ngo-1884, yakha incazelo yokuqala yesimanje yama-asidi nezisekelo ngokwemigomo yamangqamuzana.
I-Arrhenius acid ph ithiyori
Into ehlukanisayo emanzini ukuze yakhe i-hydrogen cations (H+).
I-Arrhenius isisekelo se-pH theory
Into ehlukanisayo emanzini ukuze yakhe i-hydroxide anions (OH-).
I-ARRHENIUS THEORY Iyini i-asidi? Siyini isisekelo?
I-Arrhenius acid nevidiyo eyisisekelo ye-pH theory
Ithiyori ye-Brønsted-Lowry ph
Ithini ithiyori ye-Brønsted-Lowry ye-pH?

Yahlongozwa ngo-1923 ngokuzimela ngabaseDenmark Johannes Nicolaus Bronsted kanye nesiNgisi UMartin Lowry, isekelwe embonweni we conjugate acid-base pair.
Uma i-asidi, i-HA, isabela ngesisekelo, B, i-asidi yenza isisekelo sayo se-conjugate, A.-, futhi isisekelo sakha i-conjugate acid, i-HB+, ngokushintshanisa iproton (indawo H+):
HA+B⇌A−+HB+
I-Brønsted-Lowry acid ph ithiyori
I-asidi ye-pH: ekwazi ukunikela ngama-proton (H+) ngokwesisekelo:
HA+H2O⇌A−+H3O+
Ithiyori ye-pH eyisisekelo i-Brønsted-Lowry
Into ene-pH eyisisekelo: ekwazi ukwamukela ama-proton (H+i-asidi:
B+H2O⇌HB++OH−
Le thiyori ithathwa njenge ukwenziwa jikelele yethiyori ye I-Arrhenius.
I-BRÖNSTED-LOWRY THEORY Iyini i-asidi? Siyini isisekelo?
pH theory ividiyo BRÖNSTED-LOWRY
Izincazelo zokusebenza zezilinganiso ze-pH ezingenzeka

Iyini i-ACIDITY kanye ne-ALKALINITY?
Isho ukuthini i-acidic nesisekelo se-pH?

i-asidi pH
- Okokuqala, singathola isixazululo nge-pH ene-asidi: into eshintsha iphepha le-litmus eluhlaza okwesibhakabhaka libe bomvu, isabela nezinye izinsimbi, ikhiqize usawoti futhi ikhiphe i-hydrogen (i-exothermic reaction).
- Ngaphezu kwalokho, izinto ezine-pH ene-asidi ziboleka inani eliphakathi kuka-0 no-7.
inani le-pH eliyisisekelo

- Okwesibili, kukhona I-pH yesisekelo: Into eshintsha iphepha le-litmus elibomvu libe luhlaza okwesibhakabhaka bese liphenduka libe pink lapho lisabela nge-phenolphthalein.
- Ngakolunye uhlangothi, bonisa ukuthi anenani le-pH eliphakathi kuka-7 no-14.
i-pH engathathi hlangothi

- Okokugcina, into enesilinganiso se-pH esingathathi hlangothi yileso esingaphenduli ngezinkomba ze-acid-base.
- Futhi, i-pH yalezi zinto ilingana no-7.
Izinto ezine-pH ene-asidi eqinile


Izilinganiso zezixazululo ze-asidi ku-pH
Anjani amanani ane-acidic ku-pH
- Ama-Acids akhulula ama-ion e-hydrogen, ngakho izixazululo zawo ezinamanzi ziqukethe ama-ion e-hydrogen amaningi kunamanzi angathathi hlangothi futhi athathwa njenge-acidic ngaphansi kwe-pH 7.
Yimiphi imikhiqizo evame kakhulu ye-asidi eqinile ye-pH
Kunama-asidi aqinile ayisikhombisa kuphela avamile:
- - i-hydrochloric acid HCl
- - i-nitric acid HNO3
- - i-sulfuric acid H2SO4
- - i-HBr ye-hydrobromic acid
- - I-HI hydroiodic acid
- I-perchloric acid HClO4
- - i-chloric acid HClO3

Ifomula ye-acid eqinile ye-pH
ifomula ye-asidi eqinile ye-pH
Ifomula ye-asidi eqinile ye-pH: [HNO3] = [H3O+], kanye ne-pH = -log[H3O+].
Bala i-ph inthanethi ye-asidi eqinile
Bala i-pH yesisombululo se-asidi esiqinile.
Izinto ezine-pH eyisisekelo eqinile

Izilinganiso zezixazululo eziyisisekelo ku-pH

Anjani amanani ane-acidic ku-pH
Izinto eziyisici ezine-pH eyisisekelo
- Izisekelo zamukela ama-ion e-hydrogen (zibophezela kwamanye ama-ion e-hydrogen akhiwa ukuhlukaniswa kwamanzi), ngakho izixazululo zawo ezinamanzi ziqukethe ama-ion e-hydrogen ambalwa kunamanzi angathathi hlangothi futhi zibhekwa njengeziyisisekelo ngaphezu kwe-pH 7.

Ifomula yokubala i-pH eyisisekelo eqinile
ifomula ye-asidi eqinile ye-pH
Ifomula ye-asidi eqinile ye-pH: [HNO3] = [H3O+], kanye ne-pH = -log[H3O+].
Yimiphi imikhiqizo evame kakhulu ye-asidi eqinile ye-pH
Futhi azikho izisekelo eziningi eziqinile, futhi ezinye zazo azincibiliki kakhulu emanzini. Lezo ezincibilikayo ziyi

- - i-sodium hydroxide NaOH
- - potassium hydroxide KOH
- - lithium hydroxide LiOH
- - i-rubidium hydroxide RbOH
- - i-cesium hydroxide CsOH
Ukubalwa kwesisekelo esiqinile se-pH
Ukubalwa kwesisekelo esiqinile pH
Izinto namafomula ane-asidi ebuthakathaka noma eyisisekelo

Ingakanani i-pH enamanani e-asidi / isisekelo esibuthakathaka
Isici esiyinhloko sama-asidi abuthakathaka nezisekelo ukuthi ahlukaniswe kancane emanzini. Ukulingana kuyasungulwa phakathi kwezinqubo eziya phambili nangemuva, kufinyelela esimweni esiqinile lapho izinga lokuhlukana lincike emandleni e-asidi noma isisekelo.

Ama-asidi/izisekelo ezibuthakathaka zihlukaniswa kancane emanzini. Ukuthola i-pH ye-asidi ebuthakathaka kuyinkimbinkimbi kakhulu.

I-Acid Ebuthakathaka Ifomula ye-pH
ifomula ye-pH ebuthakathaka
I-pH equation ihlala ifana: , kodwa kufanele usebenzise i i-acid dissociation njalo (Ka) ukuthola [H+].
Ifomula kaKa ithi:
kuphi: - ukuhlushwa kwe-H + ions
- ukugxila kwama-ion ayisisekelo ahlanganisiwe
- ukugcwala kwama-molecule e-asidi engahlanganisiwe
ukusabela
Bala i-pH yesisombululo se-asidi esibuthakathaka.
Bala i-pH yesisombululo se-asidi esibuthakathaka.
Isisekelo esibuthakathaka sefomula ye-pH
Ifomula yokuthola i-pH yesisekelo esibuthakathaka
Ibalwa kanjani i-pH yesisekelo esibuthakathaka?
Ngemva kokuthola i-pOH kufomula ye-pOH engenhla, i pH ungakwazi ukubala usebenzisa ifomula pH =pKw – pOH lapho pK w = 14.00.
Umehluko phakathi kwenani le-pH ne-pOH
Liyini inani le-pH elivamile?
- Ngandlela thile, i-pH iyisilinganiso sokuthi esetshenziselwa ukusungula izinga le-acidity noma i-alkalinity yesisombululo. Igama elithi “p” limele “okungenzeka”, yingakho i-pH ibizwa ngokuthi: amandla e-hydrogen.
Liyini inani le-pOH?
- Ngokwengxenye yakho. I-pOH isilinganiso sokuhlangana kwama-ion e-hydroxyl esixazululweni. Ivezwa njengesisekelo se-logarithm engu-10 ye-negative logarithm ye-hydroxyl ion concentration futhi, ngokungafani ne-pH, isetshenziselwa ukukala izinga le-alkalinity lesisombululo.
Bala isisekelo esibuthakathaka pH
Ukubalwa kwesisekelo esibuthakathaka pH
Amandla Ahlobene Ama-Acids kanye Nezisekelo

Umehluko phakathi kwe-acidic eqinile nebuthakathaka kanye ne-pH eyisisekelo
Ngabe ukuhlukaniswa kwe-pH eqinile nebuthakathaka kanye nesisekelo kuncike kuphi?
Kuya ngokuthi i-asidi i-ionized noma ihlukaniswe kanjani noma isisekelo, sihlukanisa phakathi ama-asidi/izisekelo eziqinile nezibuthakathaka, amagama achaza i- indawo ukuze ukushayela la ugesi (sibonga ukuba khona okukhulu noma okuncane kwama-ion esixazululweni).
I-ACIDI AMA-ACID AQINA KANYE ABABUTHAKATHAKA KANYE NEZISEKELO, izinga lokuhlukanisa kanye ne-pH Izibonelo
Ukuhlelwa kwe-pH ebuthakathaka ne-asidi eqinile kanye ne-bastion
Izinga le-ionization ye-acidic ne-pH eyisisekelo
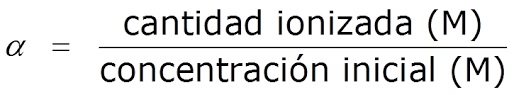
Liyini izinga le-ionization noma ukuhlukaniswa kwe-acidic ne-pH eyisisekelo
Futhi ubizwa degree of dissociation, α, ichazwa njengesilinganiso phakathi kwenani le-asidi/isisekelo se-ionized kanye nenani le-asidi/isisekelo sokuqala:
áá=inani le-asidi e-ionized/isisekelo/inani le-asidi/isisekelo sokuqala
Ngokuvamile kuvezwa njengephesenti (%).
Lisho ukuthini izinga le-ionization noma ukuhlukaniswa kwe-asidi ne-pH eyisisekelo?
ama-asidi aqinile nezisekelo
I-ionized ngokugcwele (α≈1). Bawuhambisa kahle ugesi.
- Ama-Acids: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (I-ionization yokuqala) kanye ne-HNO3.
- Izisekelo: AmaHydroksidi ensimbi ye-alkali ne-alkaline yomhlaba.
Ama-asidi nezisekelo ezibuthakathaka
I-ionized kancane: α<1. Abawuphathi kahle ugesi.
- Ama-Acids: HF(aq), H2S(aq), H2CO3, H2SO3, H3PO4, HAYI2 nama-asidi e-organic, njenge-CH3I-COOH.
- Isisekelo: NH3 (noma i-NH4OH) nezisekelo ze-nitrogenous organic, njengama-amines.
Ukuhlukaniswa okungaguquki kwe-pH acid nezisekelo
Iyini i-dissociation constant ye-pH eyisisekelo ne-acidic?
Kuyisilinganiso se amandla of a i-asidi/isisekelo kwisixazululo:
| I-ACID | BASE | |
|---|---|---|
| I-BALANCE | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| IQINISO | Ka=[A−][H3O+][HA] | KB=[HB+][OH−][B] |
| I-COLOGARHYTHM | pKa=−logKa | pKb=−logKb |
Amandla ahlobene we-acidic ne-pH eyisisekelo
I-Acidic kanye ne-pH eyisisekelo engaguquki
Ion ibhalansi yamanzi

Umthombo: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Yiziphi i-amphoteric
amphoteric ziyini
Ekhemistry, i-amphoteric substance yileyo engasabela njenge-asidi noma isisekelo..
livelaphi igama i-amphoteric
Igama lisuselwa kusiqalo sesiGreki esithi amphi- (αμφu-), okusho ukuthi 'kokubili'. Izinsimbi eziningi (ezifana ne-zinc, ithini, i-lead, i-aluminium, ne-beryllium) kanye ne-metalloids eminingi ama-oxide noma ama-hydroxides i-amphoteric.

Amanzi yi-amphiprotic substance
Kusho ukuthini ukuthi Amanzi ayi-amphiprotic substance
El amanzi kuyinto i-amphiprotic (anganikela noma amukele iproton H+), okuyivumela ukuthi isebenze njenge-asidi noma isisekelo (i-amphotericism).
Ifomula yebhalansi ye-ionic yamanzi

El i-ionic ibhalansi yamanzi ibhekisela ekuphenduleni kwamakhemikhali lapho ama-molecule amabili amanzi asabela khona ukuze akhiqize i-ion i-oxonium (H3O+) kanye ne-ion i-hydroxide (oh-):
I-equilibrium constant, ebizwa umkhiqizo we-ionic wamanzi, futhi kuchazwe ngu-Kw, kungalinganiselwa ngomkhiqizo:
Kw=[H3O+][OH−]
Ku-25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
I-pH, i-pOH kanye nomkhiqizo we-ionic wamanzi (Kw). I-ACID-BASE
Izinkomba ze-Acid-base pH

Un inkomba I-pH iyinhlanganisela yamakhemikhali i-halochromic (ishintsha umbala wayo -ukugoba- ngaphambi koshintsho ku-pH) engezwa ngamanani amancane esixazululweni ukuze kunqunywe ngokubonakalayo i-pH yayo (i-acidity noma isisekelo). Ukushintsha kombala kubizwa phenduka.
I-Litmus
Ingxube encibilikayo emanzini yodayi abahlukene ekhishwe kuyo ubulembu. Ifakwe ephepheni lokuhlunga ingenye yezinkomba ze-pH ezindala kakhulu ezisetshenziswa (∼ 1300).

I-Methyl orange
Umbala i-azo derivative lokho kuphenduka kusuka kokubomvu kuye ku-orange-yellow in i-acid medium:

I-Phenolphthalein
Isikhombi se-pH esingenambala esiphakathini se-asidi esishintsha sibe pink medium eyisisekelo:

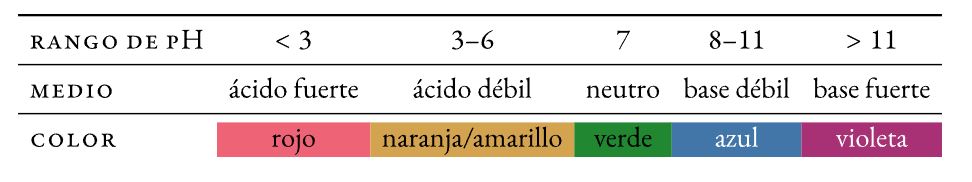
inkomba yendawo yonke
Inhlanganisela yezinkomba (i-thymol eluhlaza okwesibhakabhaka, i-methyl ebomvu, i-bromothymol eluhlaza okwesibhakabhaka, ne-phenolphthalein) ebonisa izinguquko zombala omaphakathi phezu kwenani elibanzi le-pH.
I-Acid-base neutralization titrations

I-Acid-base titration/titration iyindlela yokuhlaziya amakhemikhali amaningi
Iyini i-asidi ne-basci pH titration chemical analysis method
Una i-acid-base titration/titration kuyindlela yokuhlaziya amakhemikhali yobuningi yokunquma ukugcwala kwe-asidi ekhonjiwe noma isisekelo (hlaziya), ukuyinciphisa ncamashi ngesisombululo esijwayelekile sesisekelo noma i-asidi yokuhlushwa okwaziwayo (nesibindi).

Ijika le-titration/titration lika-25 mL ka-0.1 M acetic acid no-0.1 M i-sodium hydroxide.
I-Neutralization: ukusabela phakathi kwengxube ye-asidi nesisekelo

Kwenzekani uma uxuba i-asidi nesisekelo?
Ukusabela phakathi kwe-asidi nesisekelo kubizwa ngokuthi i-neutralization.
- Ukusabela kokungathathi hlangothi ngokuvamile kuyingozi kakhulu. lokho kusho lokho Zikhipha amandla ngendlela yokushisa.
- Se ujwayele ukukubiza ngokuthi ukungathathi hlangothi ngoba uma esabela a i-asidi nge isisekelo,
- Ngakho-ke, ukusabela phakathi kwama-asidi nezisekelo kubizwa ngokuthi i-neutralization. futhi ngaphezulu noma ngaphansi kuqeda i-acidic noma izakhiwo eziyisisekelo zazo zombili izinhlanganisela, okungukuthi, zinciphisa izakhiwo zomunye nomunye. ukukhiqiza amanzi nosawoti esikhundleni.
Ingxube ye-asidi nesisekelo iyazenzela, i-pH akudingeki ingathathi hlangothi.
- Isizathu sokuthi ingxube ye-asidi nesisekelo izenza ingathathi hlangothi i-pH akudingekile ukuba ingathathi hlangothi iyasimama ngoba yinani le-asidi kanye/noma isisekelo lapho i-pH inqunywa khona ekugcineni.
- Kunalokho, Uma inani le-H+ futhi OH- kuyefana, ikhambi lingathathi hlangothi ngoba ziyasabelana ukuze zenze amanzi (H+ +OH- →H20).
Ngokomlingiswa we-asidi kanye nesisekelo sokuphendula, amacala amane ahlukaniswa:
- I-asidi eqinile ekuqaleni + isisekelo esiqinile
- i-asidi ebuthakathaka + isisekelo esiqinile
- i-asidi eqinile + isisekelo esibuthakathaka
- Okokugcina, i-asidi ebuthakathaka + isisekelo esibuthakathaka
Kuyini ukusabela kwe-acidic nesisekelo se-pH neutralization?
Ekuphenduleni kwe ukungathathi hlangothi, i-asidi nesisekelo kusabela ngendlela efanayo -ngaphenduki ukukhiqiza usawoti namanzi:
I-ACID + BASE ⟶ USAWOTI + AMANZI
Kuye ngokuthi i-titrant iyi-asidi eqinile noma isisekelo, i-pH endaweni yokulingana izoba:
| UMHLAZIYI/INANI | qina/namandla | I-Acid Ebuthakathaka/Isisekelo Esiqinile | Isisekelo Esibuthakathaka/I-Acid Eqinile |
|---|---|---|---|
| i-pH (EQUIVALENCE) | 7 | > 7 | <7 |
| INDICATOR (ijika phakathi) | kokungathathi hlangothi | Okubalulekile | I-asidi |
Indlela Yokubala i-pH Yesixazululo

Ithini ifomula ye-pH?
Kusayensi, i-pH isilinganiso sama-ion esixazululweni. Kungase kudingeke ubale i-pH ngokusekelwe ekugxiliseni.
Ifomula yokubala i-pH
Bala i-pH usebenzisa i-pH equation: pH = -log[H3O+].
I-pH yokubala yamachibi okubhukuda
Ividiyo ibala i-pH yesisombululo
Ngo-1909, isazi samakhemikhali ezinto eziphilayo saseDenmark u-Soren Sorensen saphakamisa igama elithi pH ukuze libonise "amandla e-hydrogen ion". Uchaze i-pH njengelogarithm ethi [H+] eshintshile kuphawu. Ukuchaza kabusha njengomsebenzi we-[H3O+].
Isixazululo se-pH Calculator

I-pH yesibali Sesixazululo
Bala i-pH yesisombululo
Ngezansi kukhona izibali ezimbili ongazisebenzisa ukuze uhlole izimpendulo zezinkinga zamakhemikhali.
- Esokuqala sibala i- pH yesixazululo se i-asidi eqinile o isisekelo esiqinile.
- Futhi, eyesibili ibala i pH yesixazululo se i-asidi ebuthakathaka o isisekelo esibuthakathaka.
Bala i-pH yesisombululo esiqinile se-asidi/isisekelo
Isibali se-pH yesisombululo esiqinile se-asidi/isisekelo
[planetcalc cid=»8830″ language=»es» code=»» ilebula=»PLANETCALC, The pH of a strong acid/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]
Bala i-pH yesisombululo esibuthakathaka se-asidi/isisekelo
Isibali se-pH yesisombululo esibuthakathaka se-asidi/isisekelo
[planetcalc cid=»8834″ language=»es» code=»» ilebula=»PLANETCALC, The pH of a weak acid/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]