En Ok pool Reform, kweli candelo ngaphakathi kwe Amadama okuqubha akwinqanaba le-pH Siza kuphendula lo mbuzo ulandelayo: Ithetha ntoni i-acidic kunye nesiseko se-pH?
Isalathiso sesiqulatho sephepha
Yintoni i-pH echibini kwaye kufuneka ibe njani amanqanaba ayo?

Ithetha ukuthini ipH efanelekileyo kumadama okuqubha (7,2-7,4)
I-acronym pH imele i-hydrogen enokubakho kwaye ngumlinganiselo obonisa ubumuncu okanye ubusiseko bamanzi.
Ke I-pH ibhekisa kwisakhono se-hydrogen, ixabiso elihambelana nokuxinana kwee-ion ze-hydrogen emanzini echibini lakho kwaye ngoko ke yi-coefficient ebonisa iqondo le-asidi okanye isiseko samanzi. Ngoko ke, i-pH ijongene nokubonisa ukuxinwa kwe-H + ion emanzini, ukugqiba i-acidic okanye uphawu olusisiseko.
Ubungakanani bamaxabiso e-pH amanzi edama lokuqubha
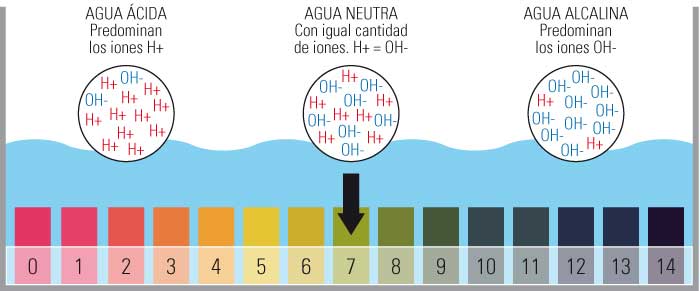

Ngawaphi amaxabiso aqukwa sisikali somlinganiselo we-pH yamanzi echibini?
- Umlinganiselo womlinganiselo we-pH ubandakanya amaxabiso ukusuka kwi-0 ukuya kwi-14.
- Ngokukodwa ukuba yi-0 eyona acidic, i-14 eyona isisiseko kunye nokubeka i-Neutral pH ku-7.
- Lo mlinganiselo umiselwa linani leeyoni ze-hydrogen zasimahla (H+) kwizinto.

Kutheni sifuna pH?
I-pH ngumlinganiselo osetyenziselwa ukucacisa ubumuncu okanye ubusiseko bomxube omanzi. Ingaba isisombululo esinamanzi siphendula njenge-asidi okanye isiseko sixhomekeke kumxholo we-hydrogen ions (H +).
Nangona kunjalo, kwanamanzi acocekileyo nangathathi hlangothi aqulethe i-hydrogen ion ngenxa yokuzahlula kwamanzi.
Kuyaziwa ukuba kwi-equilibrium phantsi kweemeko eziqhelekileyo (750 mmHg kunye ne-25 ° C), i-1 L yamanzi acocekileyo iqulethe. i-mole
y
i-mole
ion, ngoko ke, amanzi kubushushu obuqhelekileyo kunye noxinzelelo (STP) ane pH ye-7.
Yintoni omawuyenze xa i-pH yedama lethu AYIlawulwa

Yazi iziphumo ze-pH ephezulu kunye nezizathu ze-pH ephezulu echibini lakho
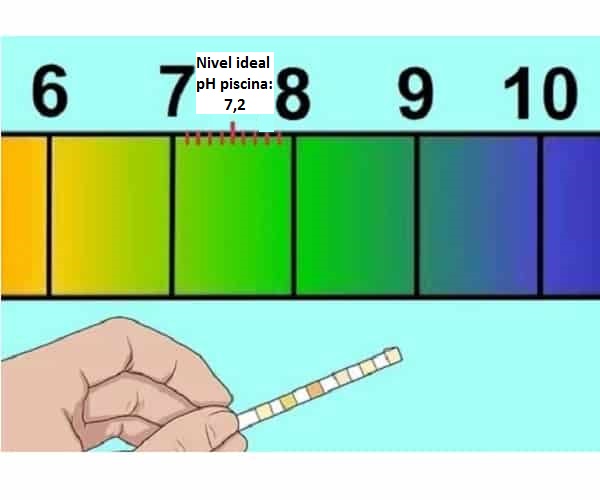
Indlela yokunyusa i-pH ye-pool kwaye kwenzekani ukuba iphantsi

Indlela yokuthoba iPool ePhakamileyo okanye eneAlkaline pH
Izikhokelo malunga nendlela yokwenza ulondolozo lwamachibi ukongeza kwi-pH: ukucocwa kwamanzi kunye nokubulala iintsholongwane

Isikhokelo esiluncedo ukwazi ukucoca idama

Isikhokelo sokugcina ichibi elinamanzi kwimeko egqibeleleyo
Inokuba njani i-pH yesisombululo?

pH yesisombululo
I-pH imele "amandla e-hydrogen" okanye "amandla e-hydrogen." I-pH yi-negative yesiseko se-10 logarithm yomsebenzi we-ion ye-hydrogen.
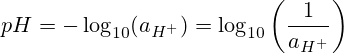
Nangona kunjalo, kwiingxaki ezininzi zeekhemikhali asisebenzisi umsebenzi we-hydrogen ion, kodwa i-molar concentration okanye i-molarity.

Zinjani izisombululo zepH ezahlukeneyo
Ukuqala, kufuneka wazi ukuba isikali se-pH yi-logarithmic.
Ngoko ke, kuthetha ukuba umahluko ngenye indlela umahluko ngokomyalelo wobukhulu, okanye amaxesha alishumi kwaye inversely ibonisa ukuxinwa ion hydrogen kwisisombululo.
Ngaloo ndlela, i-pH ephantsi ibonisa i-concentration ephezulu ye-hydrogen ion kunye ne-vice versa.

Yintoni i-asidi kunye neekhompawundi ezisisiseko kwi-pH
I-acids ezomeleleyo kunye neziseko ezomeleleyo ziyi-compounds ezithi, kuzo zonke iinjongo ezisebenzayo, zihluke ngokupheleleyo kwii-ion zabo emanzini.
Ngenxa yoko ukuxinwa kwee-ion ze-hydrogen kwizisombululo ezinjalo kunokuqwalaselwa ngokulingana nokuxinwa kwe-asidi.
Ukubala pH kuba lula
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Ukubalwa kwe-pH usebenzisa i-molar concentration ihluke kwi-asidi eqinile / isiseko kunye ne-asidi ebuthakathaka / isiseko.
I-Acidic, i-neutral kunye ne-alkaline ixabiso le-pH
Ukuhlelwa kweSikali seeMgangatho ze-pH

Ngawaphi amaxabiso e-pH

Isikali se-pH sisuka kwi-1 ukuya kwi-14, kunye ne-pH 7 isisombululo esingathathi hlangothi.
Ngoko ke, kuvela ukuba i-pH lixabiso elibonakaliswa nge-logarithmic scale phakathi kwamaxabiso 0 (i-acidic kakhulu) kunye ne-14 (i-alkaline kakhulu); Phakathi sifumana ixabiso le-7 elifakwe kwikhathalogu njengengathathi hlangothi.
Isikali se-pH isalathisi jikelele se-pH

Kuthetha ukuthini ukuba into ene-acidic okanye i-alkaline pH level?
Yintoni iiasidi kunye neziseko?
I-Acids kunye neziseko zizinto ezikhoyo kwindalo kwaye zahlulwa ngokwenqanaba le-pH, oko kukuthi, ngeqondo le-asidi okanye i-alkalinity. Ukumiselwa kokuba izinto zineasidi okanye ialkaline kulawulwa yinqanaba leasidi okanye ialkalinity elinganiswe kwisikali se-pH kunye noluhlu ukusuka ku-0 (oluneasidi kakhulu ukuya kwi-14 (i-alkaline egqithisileyo). nangona kunjalo zinemisebenzi emininzi yemizi-mveliso neyabantu.
Indlela izinto ezihlelwa ngayo ngokusekwe kwisikali samaxabiso e-pH
Ukuhlelwa kwezinto kwi-acids okanye i-alkaline ngokwexabiso le-pH
Ngokufanayo, i-acidity kunye ne-alkalinity ngamagama amabini aphendula kwindlela yokwahlula ukusabela kwayo nayiphi na into.

- Ngokufanayo, siphinda sigxininise, Isikali se-pH sisuka kwi-1 ukuya kwi-14, kunye ne-pH 7 isisombululo esingathathi hlangothi.
- Ukuba i-pH ingaphantsi kwe-7, isisombululo si-acidic., i-asidi eninzi iyancipha ixabiso le-pH ngeso sizathu a asidi yinto yekhemikhali ekwaziyo ukunikela ngeeprotons (H+) kwenye ikhemikhali.
- Ngakolunye uhlangothi, ukuba i-pH inkulu kune-7, isisombululo kuthiwa yisiseko (okanye i-alkaline) kwaye iya kuba sisiseko ngakumbi kokukhona iphezulu i-pH yayo; kwaye njengoko kubonisiwe isiseko yinto yekhemikhali ekwaziyo ukubamba iiprotons (H+) yenye imichiza.
Yintoni i-alkaline okanye isiseko ngokomlinganiselo we-pH
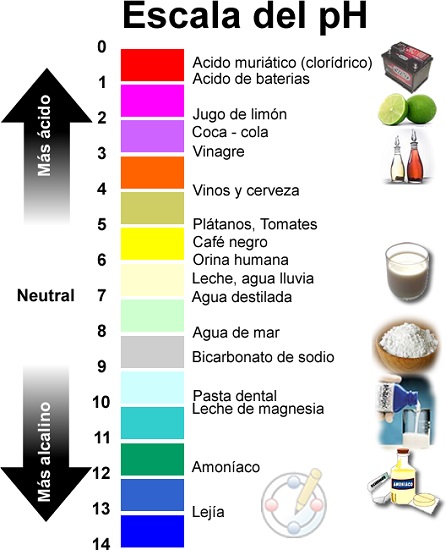
Ziziphi izinto ezineasidi?
- Inqanaba le-asidi ye-pH: pH ngaphantsi kwe-7
Kuthetha ukuthini ukuba ixabiso le-pH li-acidic?
- Ukuba into eneasidi ithetha ukuba ityebile kuH+ (i-hydrogen ions): pH ngaphezulu kwe-7
- Ngoko ke, Iiasidi zizinto ezinepH engaphantsi kwe-7. (i-pH yamanzi elingana ne-7, ithathwa njengengathathi hlangothi), i-chemistry yayo idla ngokuqulatha izixa ezikhulu ze-hydrogen ion xa ukongeza amanzi. Zidla ngokusabela nezinye izinto ngokuphulukana neeprotons (H+).
Ziziphi izinto ezingathathi hlangothi?
- Ixabiso le-pH eliphakathi: i-pH ilingana ne-7-
Kuthetha ukuthini ukuba ixabiso le-pH lingathathi hlangothi?
- I-pH ngumlinganiselo wokuba amanzi aneasidi/ayisiseko kangakanani na.
- Uluhlu luvela kwi-0 ukuya kwi-14, kunye ne-7 engathathi hlangothi.
Ziziphi izinto zealkaline?
- Izinto ezinesiseko okanye i-alkaline pH: i-pH enkulu kune-7.
Kuthetha ukuthini xa ixabiso le-pH liyi-alkaline?
- Ukuba into ethile inealkaline ithetha ukuba ihlwempuzekile kwi-H+ (okanye zizityebi kwiziseko ze-OH-, eyenza ukuba iH+).
- Kuyo yonke le nto, Iziseko, kwelinye icala, zizinto ezinepH enkulu kuno-7., ezithi kwizisombululo ezimanzi zihlala zibonelela ngeeion zehydroxyl (OH-) esiphakathini. Zidla ngokuba zii-oxidants ezinamandla, oko kukuthi, zisabela ngeeprotons ezivela kwindawo engqongileyo.
Yintoni i-asidi kunye ne-alkalinity?
Yintoni i-asidi kunye ne-alkalinity ekutyeni
Ke, kwividiyo uya kwaziswa malunga nenani elingapheliyo lokutya esikutyayo imihla ngemihla kodwa,
- Ngaba wakha wazibuza ukuba kutheni ezinye iincasa zitsala ingqalelo yethu ngakumbi kunabanye?
- Iincasa ezifana netyuwa, isonka, iziselo ezithambileyo, iijusi, neesosi.
- Ibangelwa yintoni?
- Siza kukucacisela konke oku nangaphezulu ngoku kwirekhodi.
Iithiyori ze-acidic kunye nesiseko se-pH

Iithiyori ze-Acid-base ye-pH
Yintoni i-Arrhenius pH Theory?

ecetywayo sisiswedish Svante Arrhenius ngo-1884, yenza inkcazo yokuqala yanamhlanje yeeasidi kunye neziseko ngokweemolekyuli.
Arrhenius acid ph ithiyori
Into eyahlulayo emanzini ukuze yenze ihydrogen cations (H+).
Arrhenius ithiyori esisiseko ye-pH
Into eyohlulayo emanzini ukwenza i-hydroxide anion (OH-).
ITHIYORI YE-ARRHENIUS Yintoni iasidi? Yintoni isiseko?
I-Arrhenius acid kunye nevidiyo esisiseko ye-pH yethiyori
Ithiyori ye-Brønsted-Lowry ph
Yintoni ithiyori ye-Brønsted-Lowry ye-pH?

Icetywe ngo-1923 ngokuzimeleyo yiDanish UJohannes Nicolaus Bronsted kunye nesiNgesi UMartin Lowry, isekelwe kwingcamango ye i-conjugate acid-base pairs.
Xa i-asidi, i-HA, isabela ngesiseko, i-B, i-asidi yenza isiseko sayo se-conjugate, i-A.-, kwaye isiseko senza i-asidi yayo ye-conjugate, i-HB+, ngokutshintshiselana ngeproton (ummandla we-H+):
HA+B⇌A−+HB+
I-Brønsted-Lowry acid ph ithiyori
Into ye-pH acid: ekwaziyo ukunikela ngeeprotons (H+) ngokwesiseko:
HA+H2O⇌A−+H3O+
Ithiyori esisiseko ye-pH iBrønsted-Lowry
Into ene-pH esisiseko: ekwaziyo ukwamkela iiprotons (H+) yeasidi:
B+H2O⇌HB++OH−
Le nkcazo-bungcali ithathwa njenge ngokubanzi yethiyori ye Arrhenius.
BRÖNSTED-LOWRY THEORY Yintoni iasidi? Yintoni isiseko?
pH ithiyori ividiyo BRÖNSTED-LOWRY
Iinkcazo zokusebenza zemilinganiselo ye-pH enokwenzeka

Yintoni i-ACIDITY kunye ne-ALKALINITY?
Ithetha ntoni i-acidic kunye nesiseko se-pH?

acid pH
- Kwindawo yokuqala, sinokufumana isisombululo nge-pH ene-asidi: into ejika iphepha le-litmus eluhlaza okwesibhakabhaka ibe bomvu, iphendule ngezinye isinyithi, ivelise ityuwa kwaye ikhuphe i-hydrogen (i-exothermic reaction).
- Ukongeza, izinto ezine-acidic pH ziboleka ixabiso phakathi kwe-0 kunye ne-7.
ixabiso elisisiseko pH

- Okwesibini, kukho Isiseko se-pH: Into ejika iphepha le-litmus elibomvu libe luhlaza kwaye lijike libepinki xa isabele kwiphenolphthalein.
- Kwelinye icala, bonisa ukuba zinexabiso le-pH phakathi kwe-7 kunye ne-14.
pH engathathi hlangothi

- Ekugqibeleni, into enomlinganiselo we-pH ongathathi hlangothi ngulowo ongaphenduliyo ngezalathi ze-acid-base.
- Kwakhona, i-pH yezi zinto ilingana no-7.
Izinto ezine-acidic pH eyomeleleyo


Imilinganiselo yezisombululo ze-asidi kwi-pH
Anjani amaxabiso aneasidi kwi-pH
- I-Acids ikhupha ii-ion ze-hydrogen, ngoko ke izisombululo zazo ezinamanzi ziqulethe ii-ion ze-hydrogen ezininzi kunamanzi angathathi hlangothi kwaye zithathwa njenge-asidi engaphantsi kwe-pH 7.
Ziziphi iimveliso ze-asidi ezinamandla eziqhelekileyo eziqhelekileyo ze-pH
Zisixhenxe kuphela iiasidi ezinamandla eziqhelekileyo:
- - i-hydrochloric acid HCl
- -i-nitric acid HNO3
- -iasidi yesulfuric H2SO4
- - i-hydrobromic acid HBr
- - HI hydroiodic acid
- – perchloric acid HClO4
- – iasidi chloric HClO3

Ifomula ye-asidi enamandla ye-pH
ifomula ye-asidi eqinile ye-pH
Ifomula ye-asidi enamandla ye-pH: [HNO3] = [H3O+], kunye ne-pH = -log [H3O+].
Bala i-acid eyomeleleyo kwi-intanethi
Bala i-pH yesisombululo esinamandla se-asidi.
Izinto ezine-pH esisiseko esomeleleyo

Imilinganiselo yezisombululo ezisisiseko kwi-pH

Anjani amaxabiso e-acidic kwi-pH
Izinto ezibonakalayo ezinesiseko se-pH
- Iziseko zamkela ii-ion ze-hydrogen (zibophelela kwezinye ii-ion ze-hydrogen ezenziwe ngokuhlukana kwamanzi), ngoko ke izisombululo zazo ezinamanzi zineeyoni ze-hydrogen ezimbalwa kunamanzi angathathi hlangothi kwaye zithathwa njengesiseko ngaphezulu kwe-pH 7.

Ifomula yokubala i-pH esisiseko esomeleleyo
ifomula ye-asidi eqinile ye-pH
Ifomula ye-asidi enamandla ye-pH: [HNO3] = [H3O+], kunye ne-pH = -log [H3O+].
Ziziphi iimveliso ze-asidi ezinamandla eziqhelekileyo eziqhelekileyo ze-pH
Kananjalo akukho ziseko ezininzi ezomeleleyo, kwaye ezinye zazo azinyibiliki kakhulu emanzini. Ezo zinyibilikayo zi

- -i-sodium hydroxide NaOH
- – potassium hydroxide KOH
- – lithium hydroxide LiOH
- - irubidium hydroxide RbOH
- – cesium hydroxide CsOH
Isiseko esomeleleyo sokubala pH
Ukubalwa kwesiseko esomeleleyo pH
Izinto kunye neefomyula ezine-acidic ebuthakathaka okanye i-pH esisiseko

Ingaba i-pH ixabisa njani i-asidi / isiseko esibuthathaka
Uphawu oluphambili lwee-asidi ezibuthathaka kunye neziseko kukuba zihlukaniswe ngokuyinxalenye emanzini. I-equilibrium isungulwe phakathi kweenkqubo zangaphambili kunye ne-reverse, ukufikelela kwinqanaba elizinzileyo apho iqondo lokuqhawula lixhomekeke kumandla e-asidi okanye isiseko.

Ii-asidi ezibuthathaka/iziseko zohlulwa ngokuyinxenye emanzini. Ukufumana i-pH ye-asidi ebuthathaka kunzima ngakumbi.

I-Acid ebuthathaka kwifomula ye-pH
i-acid ebuthathaka ifomula ye-pH
I-PH equation ihlala injalo: , kodwa kufuneka usebenzise i ukuhlukana kweasidi rhoqo (Ka) ukufumana [H+].
Ifomula kaKa yile:
phi: -Uxinzelelo lwe-H + ions
-Uxinzelelo lwee-ion ezisisiseko ezidibeneyo
- ukuxinwa kwee-athomu ze-asidi ezingadibaniyo
ukusabela
Bala i-pH yesisombululo esibuthathaka se-asidi.
Bala i-pH yesisombululo esibuthathaka se-asidi.
Isiseko esibuthathaka sefomula ye-pH
Ifomula yokufumana i-pH yesiseko esibuthathaka
Ibalwa njani i-pH yesiseko esibuthathaka?
Emva kokufumana i-pOH kule fomula ye-pOH ingentla, i pH unako ukubala usebenzisa ifomula pH =pKw – pOH apho pK w = 14.00.
Umahluko phakathi kwexabiso le-pH kunye ne-pOH
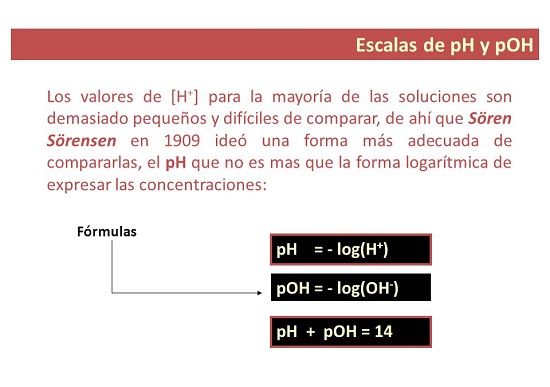
Lithini ixabiso le-pH eliqhelekileyo?
- Ngandlela ithile, i-pH ngumlinganiselo lowo isetyenziselwa ukuseka inqanaba le-asidi okanye i-alkalinity yesisombululo. I "p" imele "ithuba", yiyo loo nto i-pH ibizwa ngokuba: amandla e-hydrogen.
Yintoni ixabiso le-pOH?
- Kwelakho icala. I-pOH ngumlinganiselo wokuxinana kwee-ion ze-hydroxyl kwisisombululo. Ichazwa njengesiseko se-10 ye-logarithm engathandekiyo yoxinaniso lwe-ion ye-hydroxyl kwaye, ngokungafaniyo ne-pH, isetyenziselwa ukulinganisa inqanaba le-alkalinity yesisombululo.
Bala isiseko esibuthathaka pH
Ukubalwa kwesiseko esibuthathaka pH
Amandla anxulumeneyo e-Acids kunye neziseko

Ukwahlula phakathi kwe-acidic eqinile kunye nebuthathaka kunye nesiseko se-pH
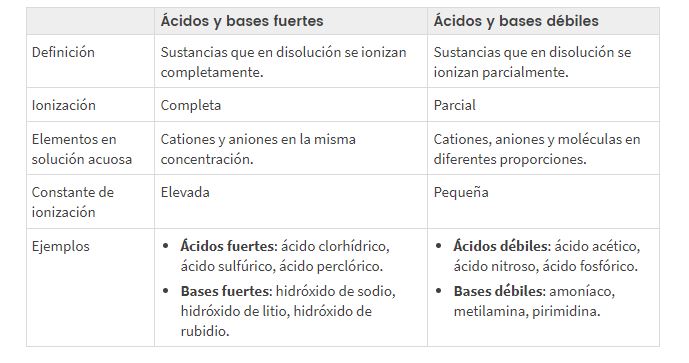
Ingaba ulwahlulo lwe-acidic eqinile kwaye ebuthathaka kunye nesiseko se-pH ixhomekeke kuyo?
Ngokuxhomekeke kwindlela i-ionized okanye i-dissociated ngayo i-asidi okanye isiseko, siyahlula phakathi iiasidi/iziseko eziqinileyo nezibuthathaka, amagama achaza i indawo for ukuqhuba la umbane (enkosi kubukho obukhulu okanye obuncinci be-ion kwisisombululo).
II-ASIDI EZAMAMANDLA KUNYE NEZISEKO, iqondo lokuqhawula kunye ne-pH Imizekelo
Ukuhlelwa kwe-pH buthathaka kunye ne-asidi eyomeleleyo kunye ne-bastion
Iqondo le-ionization ye-acidic kunye nesiseko se-pH
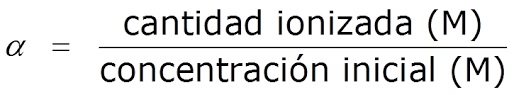
Yintoni iqondo le-ionization okanye ukuchithwa kwe-acidic kunye nesiseko se-pH
Kwabizwa kwakhona iqondo lokuzihlukanisa, α, ichazwa njengomlinganiselo phakathi komthamo we-asidi ionized/isiseko kunye nesixa se-asidi yokuqala/isiseko:
áá=ixabiso le-asidi ye-ionized/isiseko/ubungakanani be-asidi yokuqala/isiseko
Idla ngokuchazwa njengepesenti (%).
Lithetha ukuthini iqondo le-ionization okanye ukuchithwa kwe-acidic kunye nesiseko se-pH?
iiasidi ezinamandla kunye neziseko
I-ionized ngokupheleleyo (α≈1). Baqhuba kakuhle umbane.
- Iiasidi: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (I-ionization ye-1) kunye ne-HNO3.
- Iziseko: IiHydroksidi zealkali kunye neentsimbi zomhlaba zealkaline.
Iiasidi ezibuthathaka kunye neziseko
I-ionized ngokuyinxenye: α<1. Umbane awuwuphathi kakuhle.
- Iiasidi: HF(aq), H2S(aq), H2CO3, H2SO3, H3PO4HHAYI2 kunye neeasidi eziphilayo, ezifana ne-CH3COOH.
- Isiseko: NH3 (okanye NH4OH) kunye neziseko zenitrogenous eziphilayo, ezifana neeamines.
Ukuqhawula rhoqo i-pH acids kunye neziseko
Yintoni i-dissociation constant ye-pH esisiseko kunye ne-acidic?
Ngumlinganiselo we amandla a iasidi/isiseko kwisisombululo:
| I-ACID | BASE | |
|---|---|---|
| UKULINGANA | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| RHOQO | Ka=[A−][H3O+][HA] | KB=[HB+][OH−][B] |
| I-COLOGARHYTHM | pKa=−logKa | pKb=−logKb |
Amandla anxulumeneyo we-acidic kunye nesiseko se-pH
I-Acidic kunye nesiseko se-pH rhoqo
Ion ibhalansi yamanzi

Umthombo: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Yintoni amphoteric
amphoteric yintoni na
Kwi-chemistry, i-amphoteric substance yinto enokuthi iphendule njenge-asidi okanye isiseko.
livela phi igama amphoteric
Eli gama lithatyathwe kwigama lesiGrike elithi amphi- (αμφu-), elithetha 'zombini'. Iintsimbi ezininzi (ezifana ne-zinc, i-tin, ilothe, i-aluminiyam, kunye ne-beryllium) kunye ne-metalloids ezininzi iioksidi okanye iihydroxides amphoteric.

Amanzi yinxalenye ye-amphiprotic
Ithetha ukuthini into yokuba Amanzi yi-amphiprotic substance
El amanzi yinto amphiprotic (unganikela okanye wamkele iproton H+), evumela ukuba isebenze njenge-asidi okanye isiseko (i-amphotericism).
Ifomula yokulinganisela ionic yamanzi

El ionic balance yamanzi ibhekisa kwimpendulo yemichiza apho iimolekyuli ezimbini zamanzi zisabela ukuvelisa i-ion oxonium (H3O+) kunye ne-ion ihydroxide (oh-):
I-equilibrium constant, ebizwa imveliso ye-ionic yamanzi, kwaye ichazwe ngu Kw, inokuqikelelwa yimveliso:
Kw=[H3O+][OH−]
Kuma-25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
i-pH, i-pOH kunye nemveliso ye-ionic yamanzi (Kw). I-ACID-BASE
Iimpawu ze-acid-base pH

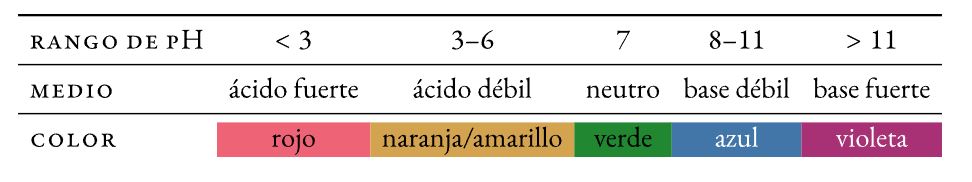
Un isalathiso I-pH yimbumba yeekhemikhali ihalochromic (utshintsha umbala wayo -ukugoba- phambi kotshintsho kwi-pH) eyongezwa kwiincinci ezincinci kwisisombululo ukwenzela ukujonga ngokubonakalayo i-pH yayo (i-acidity okanye isiseko). Ukutshintsha kombala kubizwa ngokuba jika.
I-Litmus
Umxube onyibilikayo wamanzi weedayi ezahlukeneyo ezitsalwe kuwo ubulembu. Ukufunxwa kwiphepha lokuhluza sesinye sezalathi ze-pH ezindala ezisetyenziswayo (∼ 1300).

I-Methyl orange
Imibala azo derivative leyo ijika ukusuka ebomvu ukuya kuorenji-tyheli ngaphakathi acid medium:

Phenolphthalein
Isalathisi esingenambala se-pH kwindawo yeasidi ejika ibepinki isiseko esiphakathi:

isalathisi jikelele
Umxube wezalathi (i-thymol eluhlaza okwesibhakabhaka, i-methyl ebomvu, i-bromothymol eluhlaza okwesibhakabhaka, kunye ne-phenolphthalein) ebonisa utshintsho lombala omnene kuluhlu olubanzi lwamaxabiso e-pH.
I-Acid-base neutralization titrations

I-Acid-base titration / titration yindlela yohlalutyo lweekhemikhali zobuninzi
Yintoni i-asidi kunye ne-basci pH titration indlela yohlalutyo lweekhemikhali
Omnye acid-base titration/titration yindlela yokuhlalutya imichiza yobuninzi bokumisela ukuxinwa kwe-asidi echongiweyo okanye isiseko (umhlalutyi), ukuyinciphisa ngokuthe ngqo ngesisombululo esisemgangathweni sesiseko okanye iasidi yoxinaniso olwaziwayo (nesibindi).

I-Titration / titration curve ye-25 mL ye-0.1 M i-acetic acid kunye ne-0.1 M i-sodium hydroxide.
I-Neutralization: Ukusabela phakathi komxube we-asidi kunye nesiseko

Kwenzeka ntoni ukuba udibanisa i-asidi kunye nesiseko?
Ukusabela phakathi kwe-asidi kunye nesiseko kuthiwa yi-neutralization.
- Iimpendulo zokungathathi hlangothi zidla ngokugqithisa. que kuthetha que Zinika amandla ngendlela yobushushu.
- Se udla ngokubabiza ngokuba yi neutralization kuba xa esabela a asidi nge isiseko,
- Ngoko ke, ukusabela phakathi kwe-acids kunye neziseko kubizwa ngokuba yi-neutralization. kwaye ngaphezulu okanye ngaphantsi kuphelisa i-acidic okanye iimpawu ezisisiseko zazo zombini iikhompawundi, oko kukuthi, zinciphisa iipropati zomnye nomnye. ukuvelisa amanzi kunye netyuwa endaweni yoko.
Umxube we-asidi kunye nesiseko ungathathi hlangothi ngokwawo, i-pH ayifuni ukuba ingathathi hlangothi.
- Isizathu sokuba umxube we-asidi kunye nesiseko uzithintele ngokwawo i-pH akufuneki ukuba ingathathi hlangothi iyagcinwa kuba yi-asidi kunye / okanye isiseko apho i-pH igqitywe ekugqibeleni.
- Kunoko, Ukuba inani le-H+ kunye no-OH- kuyafana, isisombululo sithatha icala kuba zisabelana ukuze zenze amanzi (H+ + OWU- →H20).
Ngokomlingiswa we-asidi kunye nesiseko sokuphendula, iimeko ezine zahlulwa:
- Iasidi eyomeleleyo ekuqaleni + isiseko esomeleleyo
- iasidi ebuthathaka + isiseko esomeleleyo
- iasidi eyomeleleyo + isiseko esibuthathaka
- Kwaye okokugqibela, i-asidi ebuthathaka + isiseko esibuthathaka
Yintoni i-acidic kunye nesiseko se-pH neutralization reaction?
Kwimpendulo ye ukungathathi hlangothi, i-asidi kunye nesiseko sisabela ngendlela efanayo ongenakulungiseka ukuvelisa ityuwa kunye namanzi:
I-ACID + ISISEKO ⟶ ITYUWA + AMANZI
Ngokuxhomekeke ekubeni i-titrant yi-asidi eyomeleleyo okanye isiseko, i-pH kwindawo yokulinganisa iya kuba:
| UHLALUTYI/ IXABISO | yomelele/yomelele | I-Acid ebuthathaka / isiseko esinamandla | Isiseko esibuthathaka / i-Acid eyomeleleyo |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| INDICATOR (ijika embindini) | Cala | Esisiseko | Iasidi |
Indlela yokubala i-pH yeSisombululo

Ithini ifomula ye-pH?
Kwinzululwazi, i-pH ngumlinganiselo weeyoni kwisisombululo. Kusenokufuneka ubale i-pH ngokusekelwe kugxininiso.
Ifomula yokubala i-pH
Bala i-pH usebenzisa i-equation ye-pH: pH = -log[H3O+].
i-pH yokubala yamadama okuqubha
Ividiyo ibala i-pH yesisombululo
Ngo-1909, isazi sezinto eziphilayo saseDenmark u-Soren Sorensen wacebisa igama elithi pH ukubonisa "ithuba le-ion ye-hydrogen". Uchaze i-pH njengelogarithm ye [H+] etshintshileyo kuphawu. Ukuchaza kwakhona njengomsebenzi we [H3O+].
Isisombululo pH Calculator

I-pH yeSikhalityhuleyitha seSisombululo
Bala i-pH yesisombululo
Ngezantsi zimbini zokubala onokuzisebenzisa ukujonga iimpendulo kwiingxaki zekhemistri.
- Eyokuqala ibala i pH yesisombululo se iasidi eyomeleleyo o isiseko esomeleleyo.
- Kwaye, eyesibini ibala i pH yesisombululo se iasidi ebuthathaka o isiseko esibuthathaka.
Bala i-pH ye-asidi eyomeleleyo / isisombululo sesiseko
Isibali-manani se-pH ye-asidi eyomeleleyo/isisombululo sesiseko
[planetcalc cid=»8830″ language=»es» code=»» label=»PLANETCALC, El pH de una solución de ácido/base fuerte» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a,#c25004″ v=»4165″]
Bala i-pH ye-asidi ebuthakathaka / isisombululo sesiseko
Isibali-manani se-pH ye-asidi ebuthathaka / isisombululo sesiseko
[planetcalc cid=»8834″ language=»es» code=»» label=»PLANETCALC, El pH de una solución de ácido/base débil» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a,#c25004″ v=»4165″]