En Ok Letamo Phetoho, karolong ena ka hare ho matamo a ho sesa a boemo ba pH Re tla sebetsana le potso e latelang: pH ea acidic le ea mantlha e bolela'ng?
Index ea litaba tsa maqephe
PH ka letamong ke eng, 'me maemo a eona a lokela ho ba joang?

pH e loketseng e bolela eng bakeng sa matamo a ho sesa (7,2-7,4)
Acronym pH e emetse hydrogen e ka bang teng mme ke tekanyo e bontshang asiti kapa motheo wa metsi.
Kahoo, pH e bolela bokhoni ba haedrojene, boleng bo tsamaellanang le bongata ba li-ion tsa haedrojene metsing a ka letamong la hau, ka hona ke coefficient e bontšang tekanyo ea asiti kapa motheo oa metsi. Ka hona, pH e ikarabella ho bonts'a bongata ba li-ion tsa H + metsing, ho khetholla sebopeho sa eona sa asiti kapa sa mantlha.
Tekanyo ea pH ea boleng ba metsi a letamo la ho sesa

Sekala sa tekanyo ea pH ea metsi a letamo se kenyelletsa litekanyetso life?
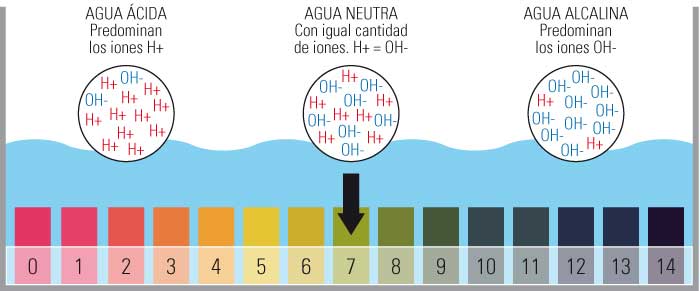
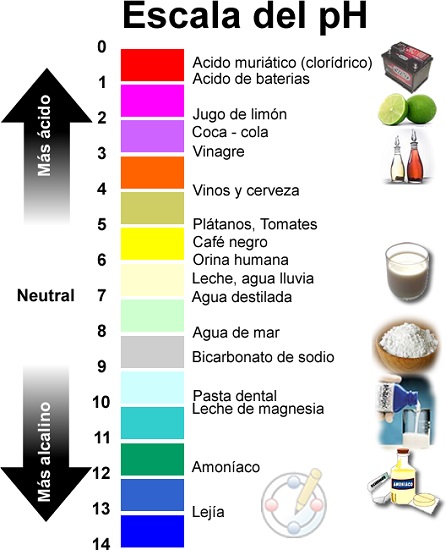
- Sekala sa tekanyo ea pH se kenyelletsa boleng ho tloha ho 0 ho isa ho 14.
- Haholo-holo ho ba 0 e nang le asiti ka ho fetisisa, 14 e le ea mantlha le ho beha Neutral pH ho 7.
- Tekanyo ena e khethoa ke palo ea li-ion tsa hydrogen (H+) tse sa lefelloeng nthong.

Ke hobane'ng ha re hloka pH?
PH ke tekanyo e sebelisetsoang ho hlalosa acidity kapa motheo oa motsoako oa metsi. Hore na tharollo e nang le metsi e sebetsa joalo ka asiti kapa setsi se ipapisitse le litaba tsa eona tsa hydrogen ion (H+).
Leha ho le joalo, esita le metsi a hloekileng a lik'hemik'hale le a sa nke lehlakore a na le li-ion tsa hydrogen ka lebaka la ho ikarola ha metsi.
Hoa tsebahala hore ka tekano tlas'a maemo a tloaelehileng (750 mmHg le 25 ° C), 1 L ea metsi a hloekileng e na le. Mol
y
Mol
ka hona, metsi a mocheso le khatello e tloaelehileng (STP) a na le pH ea 7.
Seo u lokelang ho se etsa ha pH ea letamo la rona e sa laoloe

Tseba litlamorao tse phahameng tsa pH le lisosa tsa pH e phahameng letamong la hau
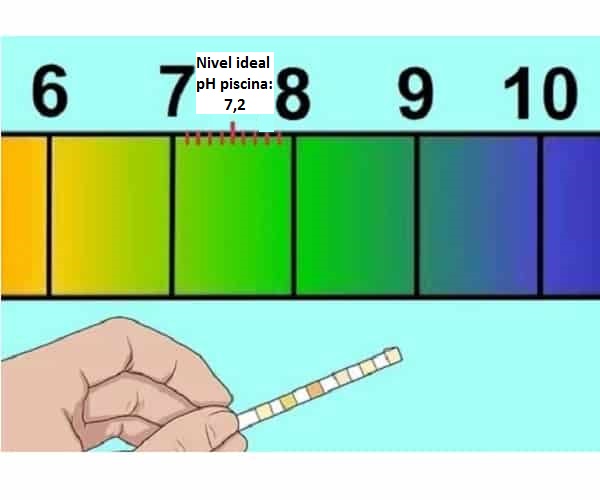
Mokhoa oa ho phahamisa pH ea letamo le ho etsahalang haeba e le tlase

Mokhoa oa ho Theola pH ea Letamo le Phahameng kapa la Alkaline
Litataiso mabapi le mokhoa oa ho lokisa letamo ho kenyelletsa pH: ho hloekisa metsi le ho bolaea likokoana-hloko

Tataiso e sebetsang ea ho tseba ho hloekisa letamo

Tataiso ea ho boloka letamo le nang le metsi maemong a phethahetseng
pH ea tharollo e ka ba joang?

pH ea tharollo
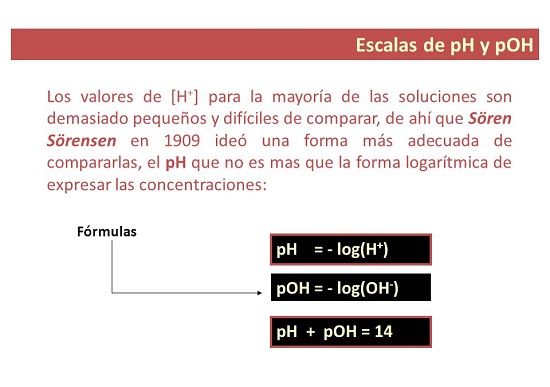
pH e emetse "bokhoni ba haedrojene" kapa "matla a haedrojene." PH ke ntho e mpe ea motheo oa 10 logarithm ea ts'ebetso ea hydrogen ion.
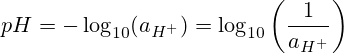
Leha ho le joalo, ka mathata a mangata a lik'hemik'hale ha re sebelise mosebetsi oa li-ion tsa haedrojene, empa mohopolo oa molar kapa molarity.

Litharollo tse fapaneng tsa pH li joang
Ho qala, o lokela ho tseba hore pH scale ke logarithmic.
Ka hona, ho bolela hore phapang ka tsela e le 'ngoe e fapane ka tatellano ea boholo, kapa makhetlo a leshome le ka tsela e fapaneng e bonts'a mahloriso a li-ion tsa hydrogen ka tharollo.
Ka hona, pH e tlase e bontša hore ho na le li-ion tsa haedrojene tse ngata le tse ling.

Ke li-acid le metsoako ea motheo ho pH
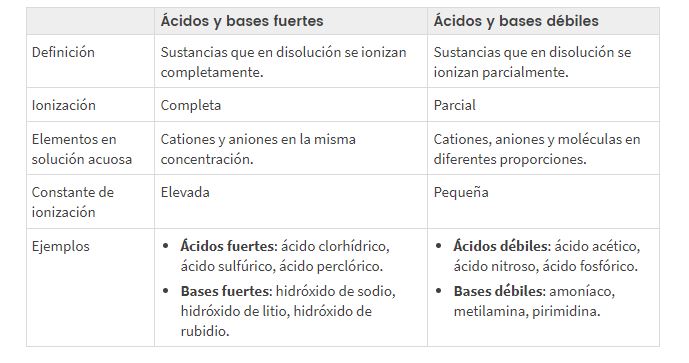
Li-acid tse matla le li-bases tse matla ke metsoako eo, bakeng sa merero eohle e sebetsang, e arohanang ka ho feletseng ho li-ion tsa tsona metsing.
Ka hona, bongata ba li-ion tsa haedrojene litharollong tse joalo bo ka nkoa bo lekana le bongata ba asiti.
Ho bala pH ho ba bonolo
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
Palo ea pH ho sebelisoa molar concentration e fapane bakeng sa asiti e matla/base le asiti e fokolang/base.
Acid, neutral le alkaline pH boleng
Tlhophiso ea Sekala sa Maemo a pH

Ke litekanyetso life tsa pH

Sekala sa pH se tloha ho 1 ho isa ho 14, 'me pH 7 e le tharollo e sa nke lehlakore.
Kahoo, ho ile ha fumaneha hore pH ke boleng bo hlahisoang ka tekanyo ea logarithmic pakeng tsa boleng 0 (e nang le asiti e feteletseng) le 14 (alkaline e feteletseng); Pakeng tsa rona re fumana boleng ba 7 bo thathamisitsoe e le boema-fofane.
pH scale universal pH indicator

Ho bolela eng hore ntho e na le pH ea acidic kapa alkaline?
Li-acids le metheo ke eng?
Li-acids le li-bases ke lintho tse teng ka tlhaho 'me li khetholloa ka boemo ba tsona ba pH, ke hore, ka tekanyo ea bona ea acidity kapa alkalinity. Qeto ea hore na lintho li na le asiti kapa alkaline e laoloa ke tekanyo ea asiti kapa alkalinity e lekantsoeng ka sekala sa pH le ho tloha ho 0 (e nang le asiti e feteletseng ho isa ho 14 (alkaline e feteletseng). Ka bobeli, hangata ke lintho tse senyang, hangata li chefo, leha ho le joalo e na le lits'ebetso tse ngata tsa indasteri le tsa batho.
Mokhoa oa ho arola likarolo ho ipapisitsoe le sekala sa boleng ba pH
Tlhophiso ea lintho ho li-acids kapa alkaline ho latela boleng ba pH
Ka mokhoa o ts'oanang, acidity le alkalinity ke mantsoe a mabeli a arabelang mokhoa oa ho arola karabelo ea ntho efe kapa efe.

- Ka mokhoa o ts'oanang, re tsitlella hape, Sekala sa pH se tloha ho 1 ho isa ho 14, 'me pH 7 e le tharollo e sa nke lehlakore.
- Haeba pH e ka tlase ho 7, tharollo e na le asiti., acid e ngata e theola boleng ba pH ka lebaka leo a asiti ke ntho ea lik'hemik'hale e khonang ho fana ka liproton (H+) ho khemikhale enngwe.
- Ka lehlakoreng le leng, haeba pH e kholo ho feta 7, tharollo e bitsoa motheo (kapa alkaline) 'me e tla ba ea bohlokoa haholo ha pH ea eona e phahame; le joalokaha ho bontšitsoe botlaaseng ke ntho ea lik'hemik'hale e khonang ho tšoara liprothone (H+) ea khemikhale enngwe.
Ke eng e nang le alkaline kapa ea motheo ho latela sekala sa pH
Lintho tse nang le asiti ke eng?
- Asiti pH boemo: pH tlase ho 7
Ho bolela eng hore pH ea boleng e na le asiti?
- Hore ntho e na le asiti ho bolela hore e ruile ka H+ (li-ion tsa haedrojene): pH e kholo ho feta 7
- Ka hona, Li-acids ke lintho tse nang le pH e ka tlase ho 7. (pH ea metsi e lekanang le 7, e nkoang e sa nke lehlakore), eo k'hemistri ea eona hangata e nang le li-ion tsa haedrojene tse ngata ha li kenya metsi. Hangata li sebetsana le lintho tse ling ka ho lahleheloa ke protons (H+).
Lintho tse sa nke lehlakore ke eng?
- Boleng ba pH bo sa nke lehlakore: pH e lekanang le 7-
Ho bolela eng hore pH ea boleng ha e nke lehlakore?
- pH ke tekanyo ea hore na metsi a na le asiti / a motheo hakae.
- Mefuta e tloha ho 0 ho isa ho 14, 'me 7 e sa nke lehlakore.
Lintho tsa alkaline ke eng?
- Lintho tse nang le pH ea motheo kapa ea alkaline: pH e kholo ho feta 7.
Ho bolela eng ha boleng ba pH e le alkaline?
- Hore ntho e na le alkaline ho bolela hore e futsanehile ho H+ (kapa e ruile metheo ea OH-, e leng se etsang hore H+).
- Bakeng sa tsena tsohle, Ka lehlakoreng le leng, metheo ke lintho tse nang le pH e kholo ho feta 7., eo ka litharollo tse nang le metsi hangata e fanang ka li-ion tsa hydroxyl (OH-) mahareng. Li atisa ho ba li-oxidants tse matla, ke hore, li itšoara ka li-proton tse tsoang sebakeng se potolohileng.
Asiti le alkalinity ke eng?
Ke eng acidity le alkalinity lijong
Joale, videong u tla tsebisoa ka palo e sa feleng ea lijo tseo re li jang letsatsi le letsatsi empa,
- Na u kile ua ipotsa hore na ke hobane'ng ha litlolo tse ling li hapa tlhokomelo ea rona ho feta tse ling?
- Litatso tse kang letsoai, bohobe, lino-mapholi, lero, esita le li-sauces.
- Hobane see ke eng?
- Re tla u hlalosetsa tsena tsohle le tse ling hona joale ha re rekota.
Likhopolo tsa acidic le tsa motheo pH

Likhopolo tsa acid-base tsa pH
Arrhenius pH Theory ke eng?

e sisintsweng ke swedish Svante Arrhenius ka 1884, e etsa tlhaloso ea pele ea sejoale-joale ea li-acids le metheo ka mantsoe a limolek'hule.
Arrhenius acid ph theory
Ntho e arohanang metsing ho etsa hydrogen cations (H+).
Arrhenius thuto ea motheo ea pH
Ntho e ikarolang ka metsing ho etsa hydroxide anions (OH-).
ARRHENIUS THEORY Asiti ke Eng? Motheo ke eng?
Arrhenius acid le video ea thuto ea motheo ea pH
Theory ea Brønsted-Lowry ph
Khopolo ea Brønsted-Lowry ea pH ke efe?

E hlahisitsoe ka 1923 e ikemetseng ke Danish Johannes Nicolaus Bronsted le Senyesemane Martin lowry, e thehiloe khopolong ea conjugate acid-base pairs.
Ha asiti, HA, e sebetsa le base, B, asiti e theha setsi sa eona sa conjugate, A.-, 'me setsi se etsa asiti ea eona ea conjugate, HB+, ka ho fapanyetsana proton (sebaka sa H+):
HA+B⇌A−+HB+
Theory ea Brønsted-Lowry acid ph
Sehlahisoa sa pH acid: se khona ho fana ka protons (H+) ho latela:
HA+H2O⇌A−+H3O+
Theory ea motheo ea pH Brønsted-Lowry
Ntho e nang le pH ea mantlha: e khona ho amohela liprothone (H+) ea acid:
B+H2O⇌HB++OH−
Khopolo ena e nkoa e le kakaretso ea khopolo ea Arrhenius.
BRÖNSTED-LOWRY THEORY Asiti ke Eng? Motheo ke eng?
pH khopolo video BRÖNSTED-LOWRY
Litlhaloso tsa ts'ebetso tsa litekanyo tsa pH tse ka khonehang

ACIDITY le ALKALINITY ke eng?
pH ea acidic le ea mantlha e bolela'ng?

acid e pH
- Ntlha ea pele, re ka fumana tharollo ka pH e nang le asiti: ntho e fetolang pampiri e putsoa ea litmus e khubelu, e sebetsana le litšepe tse ling, e hlahisa letsoai le ho lokolla hydrogen (exothermic reaction).
- Ho feta moo, lintho tse nang le acidic pH li alima boleng bo pakeng tsa 0 le 7.
boleng ba pH ea motheo

- Ea bobeli, ho na le pH ya motheo: Ntho e fetolang pampiri e kgubedu ya litmus boputswa mme e be pinki ha e sebetsanwa le phenolphthalein.
- Ka lehlakoreng le leng, bonts'a hore li na le boleng ba pH lipakeng tsa 7 le 14.
pH e sa jeleng paate

- Qetellong, ntho e nang le tekanyo ea pH e sa nke lehlakore ke e sa sebetsaneng le matšoao a acid-base.
- Hape, pH ea lintho tsena e lekana le 7.
Lintho tse nang le pH e matla ea asiti


Litekanyo tsa tharollo ea acid ka pH
Maemo a acid a joang ho pH
- Li-acid li lokolla li-ion tsa hydrogen, kahoo litharollo tsa tsona tse nang le metsi li na le li-ion tsa haedrojene tse ngata ho feta metsi a sa nke lehlakore 'me li nkoa li le acidic ka tlase ho pH 7.
Ke lihlahisoa life tse atileng haholo tsa acid e matla ea pH
Ho na le li-acid tse matla tse supileng feela tse tloaelehileng:
- - hydrochloric acid HCl
- asiti ea nitric HNO3
- - sulfuric acid H2SO4
- - hydrobromic acid HBr
- - HI hydroiodic acid
- - perchloric acid HClO4
- - chloric acid HClO3

Mokhoa o matla oa acid pH
acid e matla pH formula
Foromo e matla ea asiti ea pH: [HNO3] = [H3O+], le pH = -log[H3O+].
Bala ph online acid e matla
Bala pH ea tharollo e matla ea asiti.
Lintho tse nang le pH e matla ea motheo

Litekanyo tsa tharollo ea mantlha ho pH

Maemo a acid a joang ho pH
Lintho tse ikhethang tse nang le pH ea motheo
- Metheo e amohela li-ion tsa haedrojene (e tlama ho tse ling tsa li-ion tsa haedrojene tse entsoeng ke ho arohana ha metsi), kahoo litharollo tsa tsona tse nang le metsi li na le li-ion tsa haedrojene tse fokolang ho feta metsi a sa nke lehlakore 'me li nkoa e le tsa motheo ka holimo ho pH 7.

Foromo ea ho bala pH ea motheo e matla
acid e matla pH formula
Foromo e matla ea asiti ea pH: [HNO3] = [H3O+], le pH = -log[H3O+].
Ke lihlahisoa life tse atileng haholo tsa acid e matla ea pH
Hape ha ho na metheo e mengata e matla, 'me e meng ea eona ha e qhibilihe haholo metsing. Tse qhibilihang ke tsona

- - sodium hydroxide NaOH
- - potassium hydroxide KOH
- - lithium hydroxide LiOH
- - rubidium hydroxide RbOH
- - cesium hydroxide CsOH
Palo ea pH e matla ea motheo
Palo ea pH ea motheo e matla
Lintho le liforomo tse nang le asiti e fokolang kapa pH ea motheo

PH e na le boleng ba asiti / setsi se fokolang joang
Tšobotsi e ka sehloohong ea li-acid tse fokolang le metheo ke hore li arohane ka mokhoa o itseng metsing. Tekano e thehoa pakeng tsa mekhoa ea pele le ea morao, ho fihla boemong bo tsitsitseng boo tekanyo ea ho arohana e itšetlehileng ka matla a acid kapa setsi.

Li-acids tse fokolang / metheo e arohane hanyane ka metsing. Ho fumana pH ea asiti e fokolang ho batla ho rarahane le ho feta.

Foromo e fokolang ea Acid pH
acid e fokolang pH formula
pH equation e ntse e tšoana: , empa u tlameha ho e sebelisa ho arohana ha acid kamehla (Ka) ho fumana [H+].
Foromo ea Ka ke:
moo: - khatello ea li-ion tsa H +
- mahloriso a li-ion tsa motheo tse kopaneng
- khatello ea limolek'hule tsa acid e sa amaneng
bakeng sa karabelo
Bala pH ea tharollo e fokolang ea asiti.
Bala pH ea tharollo e fokolang ea asiti.
Foromo e fokolang ea pH ea motheo
Foromo ea ho fumana pH ea motheo o fokolang
pH ea motheo e fokolang e baloa joang?
Kamora ho fumana pOH ho tsoa ho foromo e kaholimo ea pOH, the pH u ka khona bala ho sebelisa foromo pH =pKw – pOH moo pK w = 14.00.
Phapang pakeng tsa boleng ba pH le pOH
Phihlelo e tloaelehileng ea pH ke efe?
- Ka tsela e itseng, pH ke tekanyo eo se sebedisoang ho theha boemo ba asiti kapa alkalinity ya tharollo. "p" e emetse "potential", ke ka lebaka leo pH e bitsoang: bokhoni ba hydrogen.
Boleng ba pOH ke bofe?
- Bakeng sa hau. pOH ke tekanyo ea bongata ba li-ion tsa hydroxyl ka tharollo. E hlalosoa e le motheo oa 10 negative logarithm ea hydroxyl ion concentration, 'me, ho fapana le pH, e sebelisetsoa ho lekanya boemo ba alkalinity ea tharollo.
Bala pH ea motheo e fokolang
Palo ea pH ea motheo e fokolang
Matla a Lekanyelitsoeng a Acids le Metheo

Phapang pakeng tsa acidic e matla le e fokolang le pH ea motheo
Karohano ea pH e matla le e fokolang ea asiti le ea mantlha e itšetlehile ka eng?
Ho itšetlehile ka hore na ionized kapa e arotsoe joang acid kapa setsi, re khetholla pakeng tsa li-acid tse matla le tse fokolang, mantsoe a hlalosang setsi ho khanna la motlakase (ka lebaka la boteng bo boholo kapa bo fokolang ba li-ion tharollong).
TLHOKOMELISO EA LI-ACID TSE THABANG LE TSE HLOKOMANG LE METHEO, tekanyo ea karohano le pH Mehlala
Classification pH fokola le asiti e matla le bastion
Tekanyo ea ionization ea acidic le pH ea motheo
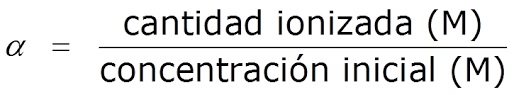
Ke tekanyo efe ea ionization kapa ho arohana ha acidic le pH ea motheo
E boetse e bitsoa tekanyo ya ho ikarola, α, e hlalosoa e le karo-karolelano pakeng tsa bongata ba asiti ea ionized / base le bongata ba asiti ea pele / motheo:
áá = palo ea asiti ea ionized / motheo / bongata ba asiti ea pele / motheo
Hangata e hlalosoa e le peresente (%).
Tekanyo ea ionization kapa dissociation ea acidic le pH ea motheo e bolela'ng?
acid e matla le metheo
E entsoe ka ionized ka botlalo (α≈1). Ba tsamaisa motlakase hantle.
- Li-acids: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (1st ionization) le HNO3.
- Metheo: Hydroxides ea alkaline le alkaline lefatše tšepe.
Li-acid tse fokolang le metheo
ionized hanyenyane: α <1. Ba tsamaisa motlakase hampe.
- Li-acids: HF(aq), H2S(aq), H2CO3, H2SO3, H3PO4, H NO2 le li-organic acid, tse kang CH3KOOHANE.
- Motheo: NH3 (kapa NH4OH) le metheo ea nitrogenous organic, joalo ka liminerale.
Dissociation kamehla pH acid le metheo
Nako ea ho arohana ea pH ea motheo le ea acidic ke eng?
Ke tekanyo ea qobella a acid/ motheo ka tharollo:
| ACID | BASE | |
|---|---|---|
| LEKELETSO | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| TS'ELISO | Ka=[A−][H3O+][HA] | KB=[HB+][OH−][B] |
| KOLOKO MOHLAKOANE | pKa=−logKa | pKb=−logKb |
Matla a lekanang a acidic le pH ea motheo
Acid le tsa motheo pH kamehla
Ion tekanyo ea metsi

Mohloli: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

Li-amphoteric ke eng
amphoteric ke eng
Ka k'hemistri, ntho ea amphoteric ke e 'ngoe e ka itšoarang joaloka acid kapa setsi..
lentsoe le tsoa kae amphoteric
Lentsoe lena le tsoa ho sehlongoapele sa Segerike amphi- (αμφu-), se bolelang 'bobeli'. Litšepe tse ngata (tse kang zinki, tin, loto, aluminium le beryllium) le metalloids tse ngata li na le oxides kapa li-hydroxides amphoteric.

Metsi ke ntho ea amphiprotic
Ho bolela eng hore Metsi ke ntho ea amphiprotic
El metsi ke ntho amphiprotic (e ka fana kapa ea amohela proton H+), e lumellang ho sebetsa joalo ka asiti kapa setsi (amphotericism).
Foromo ea ho leka-lekana ea metsi

El ionic tekanyo ea metsi e bua ka karabelo ea lik'hemik'hale eo ho eona limolek'hule tse peli tsa metsi li itšoarang ho hlahisa ione oxonium (H3O+) le ion hydroxide (Oh-):
The equilibrium constant, e bitswang sehlahisoa sa ionic sa metsi, 'me e hlalosoa ke Kw, e ka bakanyetsoa ke sehlahisoa:
Kw=[H3O+][OH−]
Ka 25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH le sehlahisoa sa ionic sa metsi (Kw). ACID-BASE
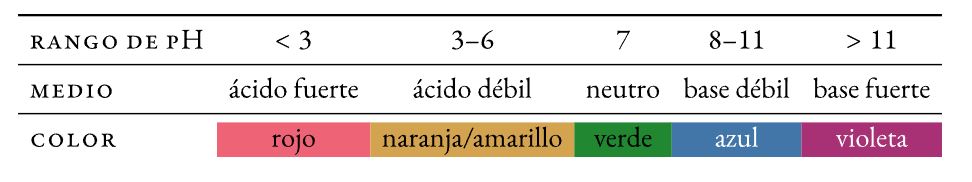
Matšoao a pH a acid-base

Un sesupo pH ke motsoako oa lik'hemik'hale halochromic (e fetola 'mala oa eona -koba- pele ho liphetoho ho pH) e kenyelletsoang ka bongata ho tharollo e le hore e bone pH ea eona (acidity kapa basicity). Phetoho ea 'mala e bitsoa reteleha.
Litmus
Motsoako o qhibilihang ka metsing oa lidae tse fapaneng tse ntšitsoeng ho boriba. E kentsoeng pampiring ea filthara ke e 'ngoe ea matšoao a khale ka ho fetisisa a pH a sebelisitsoeng (∼ 1300).

Methyl lamunu
E mebala azo derivative e fetohang ho tloha ho khubelu ho ea ho lamunu-sehla ka hare acid e mahareng:

Phenolphthalein
Sesupo sa pH se se nang mebala se mahareng a asiti se fetohang pinki seaplane sa motheo:

sesupo sa bokahohle
Motsoako oa matšoao (thymol blue, methyl red, bromothymol blue, le phenolphthalein) e bonts'ang liphetoho tsa 'mala o bonolo ho feta mefuta e mengata ea pH ea boleng.
Acid-base neutralization titrations

Acid-base titration/titration ke mokhoa oa quantitative chemical analysis
Ke eng acid le basci pH titration chemical analysis mokhoa
Una acid-base titration/titration ke mokhoa oa tlhahlobo ea lik'hemik'hale oa bongata bakeng sa ho khetholla bongata ba asiti e khethiloeng kapa setsi (analyte), ho e fokotsa hantle ka tharollo e tloaelehileng ea motheo kapa acid ea mahloriso a tsejoang (sebete).

Titration/titration curve ea 25 mL ea 0.1 M acetic acid e nang le 0.1 M sodium hydroxide.
Neutralization/ Ketso ya ho se nke lehlakore: karabelo pakeng tsa motsoako wa asiti le motheo

Ho etsahala'ng haeba u kopanya asiti le setsi?
Karabelo pakeng tsa asiti le setsi e bitsoa neutralization.
- Maikutlo a ho se nke lehlakore hangata ke a makatsang. bashagak bolela bashagak Li fana ka matla ka mokhoa oa mocheso.
- Se hangata o li bitsa neutralization hobane ha a arabela a asiti ka botlaaseng,
- Ka hona, karabelo lipakeng tsa acid le metheo e bitsoa neutralization. 'me ho feta kapa ka tlase ho felisa acidic kapa thepa ea motheo ea metsoako ka bobeli, ke hore, ba neutralize thepa ea e mong. ho hlahisa metsi le letsoai sebakeng sa.
Motsoako oa asiti le base oa itšehla thajana, pH ha ea tlameha ho se nke lehlakore.
- Lebaka la hore motsoako oa asiti le setsi se ikemisetse pH ha ea tlameha ho se nke lehlakore lea tšoarella hobane ke ka bongata ba asiti le/kapa botlaaseng moo pH e qetellang e khethiloe.
- Ho e- Haeba palo ea H+ le OH- Hoa tšoana, tharollo e fetoha e sa nke lehlakore hobane ba arabelana ho etsa metsi (H+ + OA OA- →H20).
Ho ea ka sebopeho sa acid le setsi sa ho arabela, ho na le linyeoe tse 'ne:
- Qalong asiti e matla + setsi se matla
- asiti e fokolang + setsi se matla
- asiti e matla + setsi se fokolang
- 'Me qetellong, acid e fokolang + setsi se fokolang
Karabelo ea acidic le ea mantlha ea pH neutralization ke eng?
Ka karabelo ea neutralization, acid le setsi li itšoara ka tsela e tšoanang ha e fetohe ho hlahisa letsoai le metsi:
ACID + BASE ⟶ LETSWAI + METSI
Ho itšetlehile ka hore na titrant ke acid e matla kapa setsi, pH sebakeng sa ho lekana e tla ba:
| MOHLAHLOBI/ MOHLOKOA | e matla/e matla | Asiti e fokolang / Motheo o Matla | Setsi se fokolang / Asiti e Matla |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| INDICATOR (e fetoha bohareng) | Ho se nke lehlakore | Motheo | Asiti |
Mokhoa oa ho Bala pH ea Tharollo

Foromo ea pH ke efe?
Ho saense, pH ke tekanyo ea li-ion tharollong. U ka 'na ua tlameha ho bala pH ho latela mahloriso.
Foromo ea ho bala pH
Bala pH ho sebelisa equation ea pH: pH = -log[H3O+].
pH calculator bakeng sa matamo a ho sesa
Video e bala pH ea tharollo
Ka 1909, setsebi sa biochemist sa Danish Soren Sorensen o ile a hlahisa lentsoe pH ho bontša "monyetla oa hydrogen ion". O hlalositse pH e le logarithm ea [H+] e fetohileng ka letšoao. Ho hlalosa hape joalo ka ts'ebetso ea [H3O+].
Tharollo pH Calculator

PH ea Sebali sa Tharollo
Bala pH ea tharollo
Ka tlase ho na le li-calculator tse peli tseo u ka li sebelisang ho hlahloba likarabo tsa mathata a k'hemistri.
- Ea pele e bala palo ea pH ea tharollo ea asiti e matla o motheo o matla.
- 'Me, ea bobeli e bala pH ea tharollo ea acid e fokolang o motheo o fokolang.
Bala pH ea asiti e matla/tharollo ea motheo
Khalekhuleita ea pH ea asiti e matla/tharollo ea motheo
[planetcalc cid=»8830″ language=»es» code=»» label=»PLANETCALC, The pH ea acid e matla/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]
Bala pH ea asiti e fokolang/tharollo ea motheo
Khalekhuleita ea pH ea asiti e fokolang/tharollo ea motheo
[planetcalc cid=»8834″ language=»es» code=»» label=»PLANETCALC, The pH ea acid e fokolang/base solution» colors=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]