En Ok Reform Pool, i tenei wahanga i roto i te Nga puna kaukau taumata pH Ka mahi maatau i te patai e whai ake nei: He aha te tikanga o te waikawa me te pH taketake?
Taurangi o nga ihirangi wharangi
He aha te pH i roto i te puna kaukau me pehea ona taumata?

He aha te tikanga o te pH pai mo nga puna kaukau (7,2-7,4)
Ko te acronym pH e tohu ana mo te hauwai pea, he inenga e tohu ana i te kawatanga me te maaka o te wai.
Na, Ko te pH e tohu ana ki te kaha o te hauwai, he uara e rite ana ki te kukū o nga katote hauwai i roto i te wai i roto i to puna wai, na reira ko te whakarea e tohu ana i te tohu o te kawatanga me te maatatanga o te wai. Na reira, ko te pH te kawenga mo te tohu i te kukū o nga katote H+ i roto i te wai, te whakatau i tona ahuatanga waikawa, taketake ranei.
Te tauine o nga uara pH o te wai puna kaukau
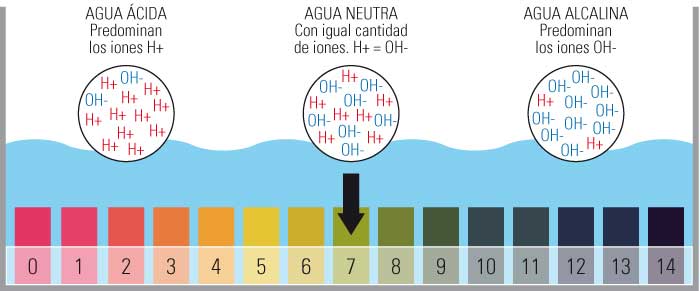

He aha nga uara kei roto i te tauine inenga pH wai puna?
- Kei roto i te tauine ine pH nga uara mai i te 0 ki te 14.
- Ina koa ko te 0 te tino waikawa, ko te 14 te mea tino taketake me te whakanoho i te pH Kupapa ki te 7.
- Ka whakatauhia tenei inenga ma te maha o nga katote hauwai (H+) kore utu i roto i te matū.

He aha tatou e hiahia ai ki te pH?
Ko te pH he inenga hei tohu i te kawatanga, i te waikore ranei o te wairewa wai. Mena ka tauhohe te wairewa wai hei waikawa, hei turanga ranei, ka whakawhirinaki ki te ihirangi o nga katote hauwai (H+).
Heoi, ahakoa te wai matū parakore me te wai kūpapa kei roto etahi katote hauwai na te wehenga-whaiaro o te wai.
E mohiotia ana i te taurite i raro i nga tikanga paerewa (750 mmHg me te 25°C), 1 rita o te wai parakore kei roto. mol
y
mol
katote, no reira, ko te wai i te pāmahana me te pehanga paerewa (STP) he pH o 7.
He aha te mahi ina KORE te pH o to tatou puna kaukau i whakaritea

Me mohio ki nga hua o te puna pH teitei me nga take o te pH teitei i roto i to puna
Me pehea te hiki ake i te pH o te puna kaukau me te aha mehemea he iti

Me pehea te Whakahekea i te pH Pool High, Alkaline ranei
He aratohu me pehea te tiaki i te puna kaukau hei taapiri i te pH: te horoi wai me te whakakorenga

He aratohu whai hua ki te mohio ki te horoi i te puna kaukau

Aratohu ki te pupuri i te puna wai ki te ahua tino pai
Me pehea te pH o te otinga?

pH o te otinga
Ko te pH e tohu ana mo te "pumanawa hauwai" ranei "te mana o te hauwai." Ko te pH te toraro o te turanga 10 haukorikori o te ngohe katote hauwai.
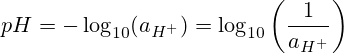
Heoi, i roto i te nuinga o nga raru matū kaore matou e whakamahi i te ngohe o nga katote hauwai, engari ko te kukū molar, molarity ranei.

He pehea te rereke o nga otinga pH
Hei timata, me mohio koe he taurangi te tauine pH.
Na reira, ko te tikanga ko te rereketanga o te rereketanga o te rereketanga o te kotahi i runga i te raupapa o te rahi, te tekau ranei nga wa me te rereke ka tohu i te kukū o nga katote hauwai i roto i te otinga.
No reira, he iti ake te pH e tohu ana he nui ake te kukū o nga katote hauwai me te rereke.

He aha te waikawa me te puhui turanga kei roto i te pH
Ko nga waikawa kaha me nga turanga kaha he pūhui e, mo nga kaupapa mahi katoa, ka wehe katoa ki o raatau katote i roto i te wai.
No reira ko te kukū o nga katote hauwai i roto i aua otinga ka taea te whakaaro he rite ki te kukū o te waikawa.
Ka ngawari te tatauranga o te pH
![pH=-log_{10}[H^+]](https://es.planetcalc.com/cgi-bin/mimetex.cgi?pH%3D-log_%7B10%7D%5BH%5E%2B%5D)
He rereke te tatauranga o te pH ma te whakamahi i te kukū molar mo te waikawa kaha/base me te waikawa/base ngoikore.
Waiwawa, kūpapa me te uara pH kawakore
Te whakarōpūtanga o te Tauine o nga Uara pH

He aha nga uara pH

Ko te tauine pH mai i te 1 ki te 14, me te pH 7 he otinga koretake.
Na, ka puta ko te pH he uara e whakaatuhia ana me te tauine arorau i waenga i nga uara 0 (tino waikawa) me te 14 (tino kawakore); I waenganui ka kitea te uara 7 kua whakarārangihia he kūpapa.
Tauine pH tohu tohu pH ao

He aha te tikanga o te matū he taumata pH waikawa, kawakore ranei?
He aha te waikawa me te turanga?
Ko nga waikawa me nga kawakore he matū kei roto i te taiao, ka tohuhia e te taumata pH, ara, ma te tohu o te waikawa, te kawakore ranei. Ko te whakatau mehemea he waikawa, he kawakore ranei nga matū ka whakatauhia e te tohu o te waikawa, te kawakoretanga ranei i inehia ma te tauine pH me te awhe mai i te 0 (tino waikawa ki te 14 (tino kawakore). ahakoa he maha nga mahi a te tangata me te ahumahi.
He pehea te whakarōpūtanga o nga huānga i runga i te tauine o nga uara pH
Whakar
Waihoki, ko te waikawa me te kawakore nga kupu e rua e whakautu ana ki te huarahi whakarōpū i te tauhohenga o tetahi huānga.

- Waihoki, ka tohe ano matou, Ko te tauine pH mai i te 1 ki te 14, me te pH 7 he otinga koretake.
- Mena he iti iho te pH i te 7, he waikawa te otinga., ka nui ake te waikawa ka iti iho te uara pH mo tera take a waikawa Ko te matū matū ka taea te koha iraoho (H+) ki tetahi atu matū.
- I tetahi atu ringa, ki te nui ake te pH i te 7, ka kiia te otinga he taketake (he kawakore ranei) a ka nui noa atu te mea ka nui ake te pH; me te mea kua whakaaturia turanga Ko te matū matū ka taea te hopu iraoho (H+) o tetahi atu matū.
He aha te kawakore, taketake ranei e ai ki te tauine pH
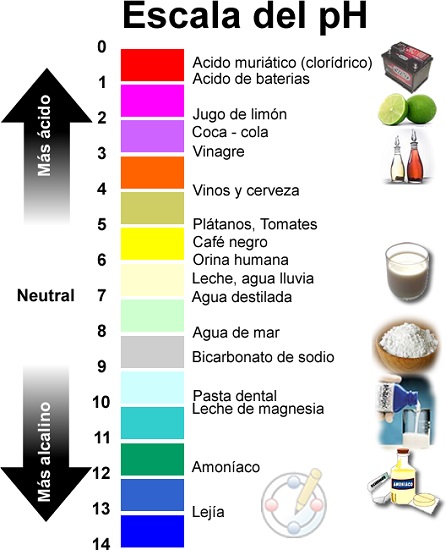
He aha nga matū waikawa?
- Te taumata pH waikawa: pH iti iho i te 7
He aha te tikanga he waikawa te uara pH?
- Ko te matū he waikawa te tikanga he nui te H+ (ngota hauwai): pH nui ake i te 7
- No reira, Ko te waikawa he matū he iti ake te pH i te 7. (pH o te wai e rite ana ki te 7, ka whakaarohia he koretake), he maha nga katote hauwai kei roto i tana matū i te taapiri wai. Ko te tikanga ka tauhohe ki etahi atu matū ma te ngaro o nga iraoho (H+).
He aha nga matū kūpapa?
- Uara pH kūpapa: pH rite ki te 7-
He aha te tikanga he koretake te uara pH?
- Ko te pH te inenga mo te waikawa/maama o te wai.
- Ko te awhe mai i te 0 ki te 14, me te 7 he koretake.
He aha nga matū kawakore?
- Ko nga matū whai pH turanga, kawakore ranei: pH nui ake i te 7.
He aha te tikanga ina he kawakore te uara pH?
- Ko te matū he kawakore te tikanga he rawakore i roto i te H+ (he taonga ranei ki nga turanga OH-, e whakakore ana i te H+).
- Mo tenei katoa, Ko nga turanga, he matū he nui ake te pH i te 7., i roto i nga wairewa wai te nuinga o te wa ka tuku nga katote hydroxyl (OH-) kei waenganui. Ko te ahua o te waikura kaha, ara, ka tauhohe ki nga iraoho mai i te reo huri noa.
He aha te waikawa me te kawakore?
He aha te waikawa me te kawakore i roto i te kai
Na, i roto i te ataata ka whakamohiohia koe mo te maha o nga kai e pau ana i ia ra, engari,
- Kua whakaaro koe he aha etahi o nga mea kakara ka nui ake te aro atu i era atu?
- Ko nga mea kakara penei i te tote, te taro, te inu ngawari, te wai, tae noa ki te ranu.
- He aha tenei?
- Ka whakamaramatia e matou enei mea katoa me etahi atu mea ki a koe inaianei i roto i te rekoata.
Nga ariā o te waikawa me te pH taketake

Nga ariā waikawa-papa o te pH
He aha te Arrhenius pH Theory?

i whakaarohia e te Swedish Svante Arrhenius i te tau 1884, ko te whakamaramatanga hou tuatahi mo te waikawa me te kawakore i roto i nga kupu ngota.
Arrhenius waikawa ph ariā
He matū ka wehe i roto i te wai hei hanga hauwai hauwai (H+).
Arrhenius taketake pH ariā
He matū ka wehe i roto i te wai hei hanga anion hydroxide (OH-).
ARRHENIUS THEORY He aha te waikawa? He aha te turanga?
Te waikawa Arrhenius me te ataata kaupapa pH taketake
Brønsted-Lowry ph ariā
He aha te ariā Brønsted-Lowry mo te pH?

I whakaarohia i te tau 1923 motuhake e te Danemaka Johannes Nicolaus Bronsted me te reo Ingarihi Matini Lowry, kei runga i te whakaaro o whakakotahi i nga takirua waikawa-papa.
Ina tauhohe tetahi waikawa, HA, ki te turanga, B, ka hanga e te waikawa tona turanga hono, A.-, ka hanga te turanga i tona waikawa conjugate, HB+, ma te whakawhiti i te proton (cation H+):
HA+B⇌A−+HB+
Brønsted-Lowry waikawa ph ariā
Waikawa pH matū: ka taea te koha iraoho (H+) ki tetahi kaupapa:
HA+H2O⇌A−+H3O+
Te ariā pH taketake Brønsted-Lowry
Te matū whai pH taketake: ka taea ki te whakaae iraoho (H+) o te waikawa:
B+H2O⇌HB++OH−
Ka whakaarohia tenei ariā he whakariterite o te ariā o Arrhenius.
TE WHAKAARO BRÖNSTED-LOWRY He aha te waikawa? He aha te turanga?
ataata ariā pH BRÖNSTED-LOWRY
Nga whakamaramatanga whakahaere mo nga inenga pH ka taea

He aha te KAUPAPA me te KAUPAPA?
He aha te tikanga o te waikawa me te pH taketake?

waikawa pH
- I te tuatahi, ka kitea he otinga ki te pH waikawa: he matū ka huri whero te pepa litmus kahurangi, ka tauhohe ki etahi konganuku, ka puta he tote me te tuku hauwai (tauhohenga exothermic).
- I tua atu, ko nga matū whai pH waikawa ka tuku uara i waenga i te 0 me te 7.
uara pH taketake

- Tuarua, kei reira nga pH turanga: He matū ka huri kahurangi te pepa litmus whero ka huri māwhero ina tauhohehia ki te phenolphthalein.
- I tetahi atu taha, tohuhia he uara pH kei waenganui i te 7 me te 14.
pH kūpapa

- Ka mutu, ko te matū he inenga pH koretake he mea karekau e aro ki nga tohu waikawa-papa.
- Ano, ko te pH o enei matū he rite ki te 7.
Nga matū whai pH waikawa kaha


Te inenga o nga wairewa waikawa i roto i te pH
He pehea nga uara waikawa i roto i te pH
- Ka tukuna e te waikawa nga katote hauwai, no reira he nui ake nga katote hauwai i o ratou wairewa wai i te wai kore, ka kiia he waikawa i raro i te pH 7.
He aha nga hua pH waikawa kaha tino noa
E whitu noa nga waikawa kaha noa:
- – waikawa pūmāota HCl
- – waikawa hauota HNO3
- – waikawa pungatara H2SO4
- – waikawa hydrobromic HBr
- – HI waikawa waikawa
- – waikawa perchloric HClO4
- – waikawa māota HClO3

Te tauira pH waikawa kaha
tātai pH waikawa kaha
Te tauira pH waikawa kaha: [HNO3] = [H3O+], me te pH = -log[H3O+].
Tātaihia te ph ipurangi waikawa kaha
Tātaihia te pH o te wairewa waikawa kaha.
Nga matū whai pH taketake kaha

Te inenga o nga otinga taketake i roto i te pH

He pehea nga uara waikawa i roto i te pH
Ko nga matū whai take pH
- Ka whakaaetia e nga turanga nga katote hauwai (ka herea ki etahi o nga katote hauwai i hangaia e te wehewehenga o te wai), no reira he iti ake nga katote hauwai i o ratou wairewa wai i te wai kore, ka kiia he mea taketake kei runga ake i te pH 7.

Tātai ki te tātai pH taketake kaha
tātai pH waikawa kaha
Te tauira pH waikawa kaha: [HNO3] = [H3O+], me te pH = -log[H3O+].
He aha nga hua pH waikawa kaha tino noa
He iti ano nga turanga pakari, ko etahi kaore e tino memeha ki te wai. Ko nga mea whakarewa he

- – konutai hydroxide NaOH
- – pāhare pāhare pāporo KOH
- – lithium hydroxide LiOH
- – rubidium hydroxide RbOH
- – cesium hydroxide CsOH
Te tatauranga pH turanga kaha
Te tatauranga o te pH turanga kaha
Ko nga matū me nga tauira he ngoikore te waikawa, te pH taketake ranei

He pehea nga uara pH waikawa / turanga ngoikore
Ko te ahuatanga matua o nga waikawa ngoikore me nga kawakore ko te wehe i tetahi waahanga ki te wai. Ka whakatauhia he taurite i waenga i nga tukanga whakamua me te whakamuri, ka eke ki te ahua pumau kei te whakawhirinaki te tohu o te wehewehe ki te kaha o te waikawa me te turanga.

Ko nga waikawa/waahanga ngoikore ka wehe noa i te wai. He uaua ake te kimi i te pH o te waikawa ngoikore.

Tauira pH Waikawa ngoikore
tātai pH waikawa ngoikore
He rite tonu te whārite pH: , engari me whakamahi koe i te te wehenga waikawa tonu (Ka) ki te kimi [H+].
Ko te tauira mo Ka ko:
kei hea: – te kukū o nga katote H+
– te kukū o nga katote turanga honohono
– te kukū o te ngota ngota ngota waikawa kore
mo te tauhohenga
Tātaihia te pH o te wairewa waikawa ngoikore.
Tātaihia te pH o te wairewa waikawa ngoikore.
Te tauira pH turanga ngoikore
Te tātai hei tiki i te pH o te turanga ngoikore
He pehea te tatau i te pH o te turanga ngoikore?
Whai muri i te whiwhi pOH mai i te tauira pOH i runga ake nei, ko te pH Ka taea e koe kiia te whakamahi i te tauira pH =pKw – pOH kei hea pK w = 14.00.
Nga rereketanga i waenga i te uara o te pH me te pOH
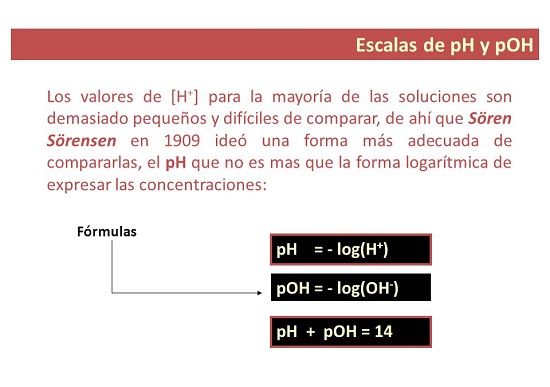
He aha te uara pH noa?
- I tetahi ara, ko te pH he ine tera whakamahia hei whakatau i te taumata o te kawakawa, te kawakoretanga ranei o te otinga. Ko te "p" e tohu ana mo te "potential", koia te take i kiia ai te pH: te kaha o te hauwai.
He aha te uara pOH?
- Mo to wahanga. Ko te pOH te inenga o te kukū o nga katote hauwai i roto i te otinga. Ka tohuhia ko te turanga 10 haukoti kino o te kukū katote hydroxyl, a, he rereke ki te pH, ka whakamahia hei ine i te taumata kawakore o te otinga.
Tātaihia te pH turanga ngoikore
Te tatauranga o te pH turanga ngoikore
Te Kaha Paanga o te Waikawa me te Papa

Te wehewehe i waenga i te kaha me te ngoikore o te waikawa me te pH taketake
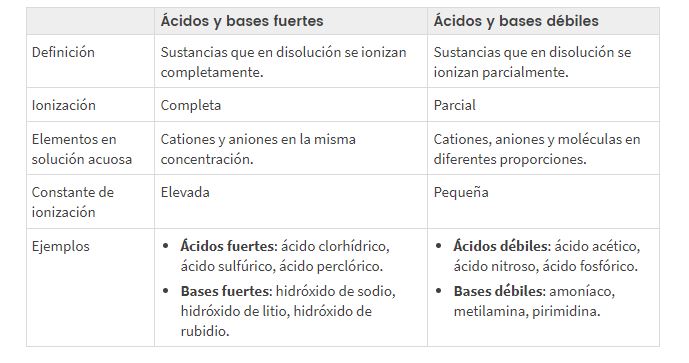
He aha te tikanga o te whakarōpūtanga o te kaha me te ngoikore o te waikawa me te pH taketake?
I runga i te ahua o te katote, te wehewehe ranei o te waikawa, te turanga ranei, ka wehewehe tatou te kaha me te ngoikore o te waikawa/base, kupu e whakaahua ana i te ngawari hoki motuka la hiko (he mihi ki te nui, iti iho ranei o nga katote i roto i te otinga).
TE KAUPAPA KAUPAPA KAUPAPA KAUPAPA KAUPAPA KAUPAPA, te tohu o te wehewehe me te pH Tauira
Te whakarōpū pH ngoikore me te waikawa kaha me te bastion
Te tohu o te katote o te waikawa me te pH taketake
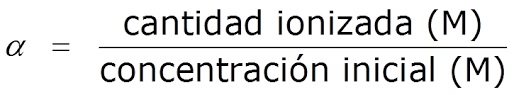
He aha te tohu o te katote, te wehewehenga ranei o te waikawa me te pH taketake
I karangahia hoki tohu o te wehewehe, α, kua tautuhia hei tauwehenga i waenga i te nui o te waikawa/tuupapa katote me te nui o te waikawa/papa tuatahi:
ááα=te nui o te waikawa katote/base/te nui o te waikawa/base tuatahi
I te nuinga o te wa ka whakaatuhia hei ōrau (%).
He aha te tohu o te katote, te wehewehenga o te waikawa me te pH taketake?
waikawa me nga turanga kaha
Kua katote katoa (α≈1). He pai ta ratou whakahaere hiko.
- Waikawa: HClO4, HI(aq), HBr(aq), HCl(aq), H2SO4 (1st ionization) me te HNO3.
- Nga turanga: Waiwai o te kawakore me nga konganuku whenua kawakore.
Te waikawa me te kawakore
He wahanga katote: α<1. He kino te whakahaere hiko.
- Waikawa: HF(aq), H2S(aq), H2CO3, H2SO3, H3PO4, H.N.O.2 me nga waikawa waro, penei i te CH3COOH.
- Taketake: NH3 (NH ranei4OH) me nga turanga waro hauota, penei i te amine.
Te wehewehe i nga waikawa me nga turanga pH
He aha te tauwehenga o te pH taketake me te waikawa?
He mehua o mālohi Tuhinga o mua waikawa/papa i roto i te otinga:
| WAWAKA | KAWAKORE | |
|---|---|---|
| PAEKE | HA+H2O⇌A−+H3O+ | B+H2O⇌HB++OH− |
| TONU | Ka=[A−][H3O+][HA] | Kb=[HB+][OH−][B] |
| KOLOGARHYTHM | pKa=−logKa | pKb=−logKb |
Te kaha o te waikawa me te pH taketake
Waikawa me te pH taketake tonu
Taurite katote o te wai

Puna: https://commons.wikimedia.org/wiki/File:Autoionizacion-agua.gif

He aha nga amphoteric
amphoteric he aha enei
I roto i te matū, he matū amphoteric tetahi ka taea te tauhohe hei waikawa, hei turanga ranei.;
no hea te kupu amphoteric
I ahu mai te kupu i te kupu Kariki prefix amphi- (αμφu-), te tikanga 'e rua'. He maha nga konganuku (pēnei i te konutea, te tine, te mata, te konumohe, me te beryllium) me te nuinga o nga konganuku waikura waiwai ranei amphoteric.

Ko te wai he matū amphiprotic
He aha te tikanga he matū amphiprotic te wai
El wai he matū amphiprotic (ka taea te koha, te whakaae ranei i tetahi proton H+), e taea ai te mahi hei waikawa, hei turanga ranei (amphotericism).
tātai taurite katote wai

El toenga katote o te wai e pā ana ki te tauhohenga matū e tauhohe ai ngā rāpoi ngota wai e rua ki te whakaputa katote oxonium (H3O+) me te katote hāora (ō-):
Ko te taurite tonu, ka kiia hua katote o te wai, me te tohu e Kw, ka taea te tata ki te hua:
Kw=[H3O+][OH−]
I te 25°C:
[H3O+]=[OH−]=10−7M⇒Kw=10−14
pH, pOH me te hua katote o te wai (Kw). KAUPAPA KAUPAPA
Nga tohu pH waikawa-papa

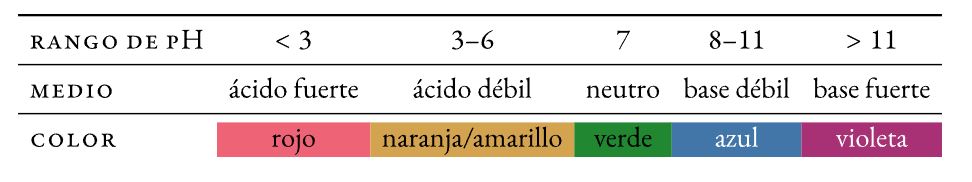
Un tohu He pūhui matū te pH halochromic (ka huri i tona tae -whakapiko— i mua i nga huringa o te pH) ka taapirihia ki roto i nga rahinga iti ki te otinga kia kitea ai te ahua o tona pH (te kawawawatanga, te waitake ranei). Ko te huringa tae ka kiia huri.
Ritihi
Te ranunga wairewa o nga tae rereke ka tangohia mai i raihana. Ka horomia ki te pepa tātari ko tetahi o nga tohu pH tawhito rawa i whakamahia (∼ 1300).

Karaka Methyl
Tihoko pärönaki azo ka huri mai i te whero ki te karaka-kowhai reo waikawa:

Phenolphthalein
Tohu pH kore tae i roto i te reo waikawa ka huri mawhero reo taketake:

tohu ao
Whakaranuhia nga tohu (thymol blue, methyl red, bromothymol blue, and phenolphthalein) e whakaatu ana i nga huringa tae ngawari ki runga i te whānuitanga o nga uara pH.
Te whakaheke i te waikawa-papaku

Ko te titrate waikawa-papa he tikanga mo te tātari matū ine
He aha te waikawa me te basci pH titration tikanga tātari matū
una te titration acid-base he tikanga tātari matū ine ine hei whakatau i te kukū o te waikawa, te turanga ranei (tātari), ka whakakorehia ki te otinga paerewa o te turanga, te waikawa ranei o te kukū e mohiotia ana (toa).

Te anau titration/titration o te 25 mL o te 0.1 M waikawa ahetiki me te 0.1 M te konutai waikawa.
Te whakakore: tauhohenga i waenga i te ranunga o te waikawa me te turanga

Ka aha ki te whakaranu koe i te waikawa me te kawakore?
Ko te tauhohenga i waenga i te waikawa me te kawakore ka kiia ko te whakakore.
- Ko nga tauhohenga whakangao he mea whakawera. e te tikanga e Ka tukuna e ratou te kaha ki te ahua o te wera.
- Se Ko te tikanga ka kiia e ia ko te whakahekenga na te mea ka tauhohe a waikawa me te turanga,
- Na reira, ko te tauhohenga i waenga i te waikawa me te kawakore e kiia ana ko te whakaheke. ka nui ake, iti iho ranei ka whakakore i nga huatanga waikawa, taketake ranei o nga puhui e rua, ara, ka whakakore i nga ahuatanga o tetahi ki tetahi. te whakaputa wai me te tote.
Ko te ranunga o te waikawa me te turanga ka whakakore i a ia ano, karekau te pH kia noho koretake.
- Ko te take i whakakorehia ai e te ranunga o te waikawa me te kawakore te pH kia kore e noho koretake ka mau tonu na te mea ma te nui o te waikawa me te turanga ka whakatauhia te pH.
- Engari, Mena ko te nui o te H+ me OH- he rite tonu, ka noho koretake te otinga na te mea ka tauhohe raua ki a raua ki te hanga wai (H+ +OH- →H20).
I runga i te ahua o te waikawa me te turanga tauhohenga, e wha nga keehi ka wehea:
- I te timatanga ko te waikawa kaha + te turanga kaha
- waikawa ngoikore + turanga kaha
- waikawa kaha + turanga ngoikore
- Ka mutu, ko te waikawa ngoikore + te turanga ngoikore
He aha te tauhohenga whakaheke pH waikawa me te taketake?
I roto i te tauhohenga o whakakorenga, he rite tonu te tauhohenga o te waikawa me te kawakore e kore e taea te whakahoki ki te whakaputa i te tote me te wai:
WAIWA + PUKA ⟶ TOTE + WAI
I runga i te mea he waikawa kaha, he kawau ranei te titant, ko te pH i te ira taurite ko:
| KAUPAPA KAUPAPA | kaha/kaha | Waikawa ngoikore/Tuapapa Kaha | Papa ngoikore/Wakawa Kaha |
|---|---|---|---|
| pH (EQUIVALENCE) | 7 | > 7 | <7 |
| TOHUTOHU (ka huri ki waenganui) | kūpapa | Tuhinga | Waikawa |
Me pehea te Tatau i te pH o te Otinga

He aha te tauira mo te pH?
I roto i te pūtaiao, ko te pH te ine o nga katote i roto i te otinga. Me tatau pea koe i te pH i runga i te kukū.
Tātai mō te tātai pH
Tātaihia te pH mā te whakamahi i te whārite pH: pH = -log[H3O+].
Te tatauranga pH mo nga puna kaukau
Ataata tatau te pH o te otinga
I te tau 1909, ka whakaarohia e te tohunga koiora Danish a Soren Sorensen te kupu pH hei tohu i te "pumanawa o te katote hauwai". I tautuhia e ia te pH ko te taukohiko o te [H+] i huri i te tohu. Te tautuhi ano hei mahi a [H3O+].
Whakataunga pH Calculator

Ko te pH o te Kaitito Whakataunga
Tātaihia te pH o tētahi otinga
Kei raro nei e rua nga taatai ka taea e koe te whakamahi ki te tirotiro i nga whakautu ki nga raru matū.
- Ko te tuatahi ka tatau i te pH o te otinga o waikawa kaha o turanga pakari.
- Na, ko te tuarua ka tatau i te pH o te otinga o waikawa ngoikore o turanga ngoikore.
Tātaihia te pH o te wairewa waikawa/papa kaha
He tātaitai mo te pH o te wairewa waikawa/papa kaha
[planetcalc cid=»8830″ language=»es» code=»» label=»PLANETCALC, Ko te pH o te waikawa kaha/wairewa turanga» tae=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]
Tātaihia te pH o te wairewa waikawa/waiwai ngoikore
He tātaitai mo te pH o te wairewa waikawa/papa ngoikore
[planetcalc cid=»8834″ language=»es» code=»» label=»PLANETCALC, Te pH o te wairewa waikawa/base ngoikore» tae=»#263238,#435863,#090c0d,#fa7014,#fb9b5a, # c25004″ v=»4165″]